Abstract
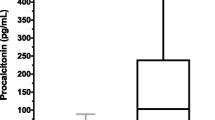
In human medicine, procalcitonin (PCT) is a very common and well-established biomarker for sepsis. Even though sepsis is also a leading cause of death in foals and adult horses, up to now, no data about the role of equine PCT in septic horses has been available. Based on monoclonal antibodies targeted against human PCT, we report here the development of a sandwich ELISA for the quantification of equine PCT in equine plasma samples. The ELISA was characterized for intra- and interassay variance and a working range from 25 to 1,000 ng mL−1 was defined as within this range; both intra- and interassay variances were below 15 %. The target recovery ranged between 73 and 106 %. The ELISA was used to determine the equine PCT concentration in 24 healthy and 5 septic horses to show the potential for clinical evaluation of equine PCT. Significantly different (P = 0.0006) mean equine PCT concentrations were found for the healthy control group and the sepsis group (47 and 8,450 ng mL−1).


Similar content being viewed by others
References
Sykes B, Furr M (2005) Equine endotoxaemia-a state of the art review of therapy. Aust Vet J 83(January, Febuary 2005):45–49
King J, Gerring E (1988) Detection of endotoxemia in cases of equine colic. Vet Rec 123:269–271
Moore J, Barton M (1998) An update on endotoxemia. Part 1. Mechanisms and pathways. Equine Vet Educ 10:300–306
Moore J, White N, Berg JN et al (1981) Endotoxemia following experimental intestinal strangulation obstruction in ponies. Can J Comp Med 45:330–332
Beadle R, Huber T (1977) Blood chemistry changes associated with rapid intravenous administration of Escherichia coli endotoxin in anesthetized ponies. J Equine Med Surg 1:371–375
Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C (1993) High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 341(8844):515–518
Schneider H, Lam QT (2007) Procalcitonin for the clinical laboratory: a review. Pathology 39:383–390
Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34(6):1589–1596. doi:10.1097/01.ccm.0000217961.75225.e9
Toribio RE, Kohn CW, Leone GW, Capen CC, Rosol TJ (2003) Molecular cloning and expression of equine calcitonin, calcitonin gene-related peptide-I, and calcitonin gene-related peptide-II. Mol Cell Endocrinol 199(1–2):119–128. doi:10.1016/s0303-7207(02)00289-7
Giunti M, Gentilini F, Sanguinetti V, Famigli P, Bergamini (2006) SIRS increases circulating procalcitonin in dogs. Shock 25:73. doi:10.1097/00024382-200606001-00222
Holowaychuk MK, Martin LG (2007) Review of hypocalcemia in septic patients. J Vet Emerg Crit Care 17(4):348–358. doi:10.1111/j.1476-4431.2007.00246.x
Redl H, Schiesser A, Togel E, Assicot M, Bohuon C (2001) Possible role of TNF on procalcitonin release in a baboon model of sepsis. Shock 16(1):25–27. doi:10.1097/00024382-200116010-00005
Wagner KE, Martinez JM, Vath SD, Snider RH, Nylen ES, Becker KL, Muller B, White JC (2002) Early immunoneutralization of calcitonin precursors attenuates the adverse physiologic response to sepsis in pigs. Crit Care Med 30(10):2313–2321. doi:10.1097/01.ccm.0000030446.45432.43
Pusterla N, Magdesian KG, Mapes S, Leutenegger CM (2006) Expression of molecular markers in blood of neonatal foals with sepsis. Am J Vet Res 67(6):1045–1049. doi:10.2460/ajvr.67.6.1045
Breuer J, Schusser GF (2012) Establishing a sepsis-score for adult equine patients. Pferdeheilkunde 28(4):421–428
Kremmer E, Meyer K, Graesser FA, Flatley A, Koesters M, Luppa PB, Kraemer PM (2012) A new strategy for the development of monoclonal antibodies for the determination of human procalcitonin in serum samples. Anal Bioanal Chem 402(2):989–995. doi:10.1007/s00216-011-5475-4
Rascher D, Geerlof A, Kremmer E, Krämer P, Schmid M, Hartman A, Rieger M (2014) Total internal reflection (TIRF)-based quantification of procalcitonin for sepsis diagnosis—a point-of-care testing application. Biosens Bioelectron. doi:10.1016/j.bios.2014.03.052
Studier FW (2005) Protein production by auto-induction in high-density shaking cultures. Protein Expr Purif 41(1):207–234. doi:10.1016/j.pep.2005.01.016
Marblestone JG, Edavettal SC, Lim Y, Lim P, Zuo X, Butt TR (2006) Comparison of SUMO fusion technology with traditional gene fusion systems: enhanced expression and solubility with SUMO. Protein Sci 15(1):182–189. doi:10.1110/ps.051812706
FDA (2013) Guidance for industry: bioanalytical method validation
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 829 kb)
Rights and permissions
About this article
Cite this article
Rieger, M., Kochleus, C., Teschner, D. et al. A new ELISA for the quantification of equine procalcitonin in plasma as potential inflammation biomarker in horses. Anal Bioanal Chem 406, 5507–5512 (2014). https://doi.org/10.1007/s00216-014-7944-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-7944-z