Abstract
Summary
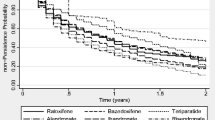
Little is known about treatment patterns with injectable osteoporosis therapies. At 12 months, the probability of discontinuation was 69.1% among patients using ibandronate, followed by teriparatide (67.1%), zoledronic acid (59.2%), and denosumab (48.8%). By 24 months, discontinuation was higher for each treatment. The majority of US patients discontinue injectable osteoporosis treatment by the end of the first year following initiation.
Introduction
This study was designed to assess the frequency of treatment discontinuation over time among patients who initiate injectable osteoporosis therapies.
Methods
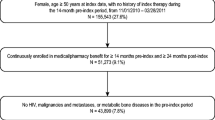
This retrospective observational study utilized an administrative claims database to measure discontinuation of injectable osteoporosis therapy, reported at 6-month intervals over 2 years. Eligible patients were aged ≥55 years, had newly initiated injectable osteoporosis therapy between January 2008 and June 2012, and were continuously enrolled in the health plan for ≥1 year prior to and ≥1.5 years after the date the first injectable medication was received (the index date). Follow-up time ranged from 18 to 24 months. Injectable osteoporosis treatments included in the analysis were denosumab, ibandronate, teriparatide, and zoledronic acid. Discontinuation was assessed using Kaplan–Meier survival analysis and was defined at each time point as the percentage of patients who did not receive the dose scheduled for that time point. A 90-day grace period was allowed to accommodate flexibility in the scheduling of post-index re-administrations. Sensitivity analyses assessed discontinuation using grace periods of 60 and 30 days.
Results
A total of 4756 patients met the inclusion criteria for the study, with 617 utilizing denosumab, 233 ibandronate, 778 teriparatide, and 3128 zoledronic acid. At 12 months, discontinuation was highest among patients using ibandronate (69.1%), followed by teriparatide (67.1%), zoledronic acid (59.2%), and denosumab (48.8%). By 24 months, discontinuation was higher for each treatment: 87.5% for ibandronate, 87.9% for teriparatide, 79.8% for zoledronic acid, and 64.3% for denosumab.
Conclusions
The majority of US patients discontinue injectable osteoporosis treatment by the end of the first year following initiation.



Similar content being viewed by others
References
Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK (2008) Medication compliance and persistence: terminology and definitions. Value Health 11:44–47. doi:10.1111/j.1524-4733.2007.00213.x
Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ (2007) Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc 82:1493–1501. doi:10.1016/s0025-6196(11)61093-8
Karlsson L, Lundkvist J, Psachoulia E, Intorcia M, Strom O (2015) Persistence with denosumab and persistence with oral bisphosphonates for the treatment of postmenopausal osteoporosis: a retrospective, observational study, and a meta-analysis. Osteoporos Int 26:2401–2411. doi:10.1007/s00198-015-3253-4
Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, Silverman S (2006) Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 81:1013–1022. doi:10.4065/81.8.1013
Caro JJ, Ishak KJ, Huybrechts KF, Raggio G, Naujoks C (2004) The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int 15:1003–1008. doi:10.1007/s00198-004-1652-z
Huybrechts KF, Ishak KJ, Caro JJ (2006) Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone 38:922–928. doi:10.1016/j.bone.2005.10.022
Boonen S, Vanderschueren D, Venken K, Milisen K, Delforge M, Haentjens P (2008) Recent developments in the management of postmenopausal osteoporosis with bisphosphonates: enhanced efficacy by enhanced compliance. J Intern Med 264:315–332. doi:10.1111/j.1365-2796.2008.02010.x
Das S, Crockett JC (2013) Osteoporosis—a current view of pharmacological prevention and treatment. Drug Des Devel Ther 7:435–448. doi:10.2147/dddt.s31504
Reginster JY, Rabenda V (2006) Patient preference in the management of postmenopausal osteoporosis with bisphosphonates. Clin Interv Aging 1:415–423
Papaioannou A, Kennedy CC, Dolovich L, Lau E, Adachi JD (2007) Patient adherence to osteoporosis medications: problems, consequences and management strategies. Drugs Aging 24:37–55
Ringe JD, Farahmand P (2014) Improved real-life adherence of 6-monthly denosumab injections due to positive feedback based on rapid 6-month BMD increase and good safety profile. Rheumatol Int 34:727–732. doi:10.1007/s00296-012-2663-2
Briot K, Ravaud P, Dargent-Molina P, Zylberman M, Liu-Leage S, Roux C (2009) Persistence with teriparatide in postmenopausal osteoporosis; impact of a patient education and follow-up program: the French experience. Osteoporos Int 20:625–630. doi:10.1007/s00198-008-0698-8
Hazel-Fernandez L, Louder AM, Foster SA, Uribe CL, Burge RT (2013) Association of teriparatide adherence and persistence with clinical and economic outcomes in Medicare part D recipients: a retrospective cohort study. BMC Musculoskelet Disord 14:4. doi:10.1186/1471-2474-14-4
Bonafede MM, Shi N, Bower AG, Barron RL, Grauer A, Chandler DB (2015) Teriparatide treatment patterns in osteoporosis and subsequent fracture events: a US claims analysis. Osteoporos Int 26:1203–1212. doi:10.1007/s00198-014-2971-3
Yu S, Burge RT, Foster SA, Gelwicks S, Meadows ES (2012) The impact of teriparatide adherence and persistence on fracture outcomes. Osteoporos Int 23:1103–1113. doi:10.1007/s00198-011-1843-3
Foster SA, Foley KA, Meadows ES, Johnston JA, Wang SS, Pohl GM, Long SR (2011) Adherence and persistence with teriparatide among patients with commercial, Medicare, and Medicaid insurance. Osteoporos Int 22:551–557. doi:10.1007/s00198-010-1297-z
Kyvernitakis I, Kostev K, Kurth A, Albert US, Hadji P (2014) Differences in persistency with teriparatide in patients with osteoporosis according to gender and health care provider. Osteoporos Int 25:2721–2728. doi:10.1007/s00198-014-2810-6
Lee YK, Nho JH, Ha YC, Koo KH (2012) Persistence with intravenous zoledronate in elderly patients with osteoporosis. Osteoporos Int 23:2329–2333. doi:10.1007/s00198-011-1881-x
Hadji P, Papaioannou N, Gielen E, Feudjo Tepie M, Zhang E, Frieling I, Geusens P, Makras P, Resch H, Moller G, Kalouche-Khalil L, Fahrleitner-Pammer A (2015) Persistence, adherence, and medication-taking behavior in women with postmenopausal osteoporosis receiving denosumab in routine practice in Germany, Austria, Greece, and Belgium: 12-month results from a European non-interventional study. Osteoporos Int 26:2479–2489. doi:10.1007/s00198-015-3164-4
Curtis JR, Yun H, Matthews R, Saag KG, Delzell E (2012) Adherence with intravenous zoledronate and intravenous ibandronate in the United States Medicare population. Arthritis Care Res (Hoboken) 64:1054–1060. doi:10.1002/acr.21638
Silverman SL, Siris E, Kendler DL, Belazi D, Brown JP, Gold DT, Lewiecki EM, Papaioannou A, Simonelli C, Ferreira I, Balasubramanian A, Dakin P, Ho P, Siddhanti S, Stolshek B, Recknor C (2015) Persistence at 12 months with denosumab in postmenopausal women with osteoporosis: interim results from a prospective observational study. Osteoporos Int 26:361–372. doi:10.1007/s00198-014-2871-6
Cheng LI, Durden E, Limone B, Radbill L, Juneau PL, Spangler L, Mirza FM, Stolshek BS (2015) Persistance and compliance with osteroporosis therapies among women in a commercially insured population in the United States. J Manag Care Spec Pharm 21:824–833
Papaioannou A, Khan A, Belanger A, Bensen W, Kendler D, Theoret F, Amin M, Brekke L, Erdmann M, Walker V, Adachi JD (2015) Persistence with denosumab therapy among osteoporotic women in the Canadian patient-support program. Curr Med Res Opin 31:1391–1401. doi:10.1185/03007995.2015.1053049
Cornell University Law School 45 CFR 164.512 Uses and disclosures for which an authorization or opportunity to agree or object is not required. https://www.law.cornell.edu/cfr/text/45/164.512. Accessed June 29 2016
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Kanis JA, Cooper C, Hiligsmann M, Rabenda V, Reginster JY, Rizzoli R (2011) Partial adherence: a new perspective on health economic assessment in osteoporosis. Osteoporos Int 22:2565–2573. doi:10.1007/s00198-011-1668-0
Lo JC, Pressman AR, Omar MA, Ettinger B (2006) Persistence with weekly alendronate therapy among postmenopausal women. Osteoporos Int 17:922–928. doi:10.1007/s00198-006-0085-2
Weycker D, Macarios D, Edelsberg J, Oster G (2007) Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int 18:271–277. doi:10.1007/s00198-006-0230-y
Cramer JA, Gold DT, Silverman SL, Lewiecki EM (2007) A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 18:1023–1031. doi:10.1007/s00198-006-0322-8
Siris ES, Selby PL, Saag KG, Borgstrom F, Herings RM, Silverman SL (2009) Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med 122:S3–13. doi:10.1016/j.amjmed.2008.12.002
van Boven JF, de Boer PT, Postma MJ, Vegter S (2013) Persistence with osteoporosis medication among newly-treated osteoporotic patients. J Bone Miner Metab 31:562–570. doi:10.1007/s00774-013-0440-2
Fan T, Zhang Q, Sen SS (2013) Persistence with weekly and monthly bisphosphonates among postmenopausal women: analysis of a US pharmacy claims administrative database. Clinicoecon Outcomes Res 5:589–595. doi:10.2147/ceor.s39076
Tamone C, Fonte G, Panico A, Molinatti PA, D'Amelio P, Isaia GC (2012) Impact of a phone follow-up program on persistence with teriparatide or PTH(1-84) treatment. Calcif Tissue Int 90:272–278. doi:10.1007/s00223-012-9574-9
Fuksa L, Vytrisalova M (2015) Adherence to denosumab in the treatment of osteoporosis and its utilization in the Czech Republic. Curr Med Res Opin 31:1645–1653. doi:10.1185/03007995.2015.1065241
Penning-van Beest FJ, Goettsch WG, Erkens JA, Herings RM (2006) Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther 28:236–242. doi:10.1016/j.clinthera.2006.01.002
Cotte FE, Fardellone P, Mercier F, Gaudin AF, Roux C (2010) Adherence to monthly and weekly oral bisphosphonates in women with osteoporosis. Osteoporos Int 21:145–155. doi:10.1007/s00198-009-0930-1
Carr AJ, Thompson PW, Cooper C (2006) Factors associated with adherence and persistence to bisphosphonate therapy in osteoporosis: a cross-sectional survey. Osteoporos Int 17:1638–1644. doi:10.1007/s00198-006-0166-2
Arden NK, Earl S, Fisher DJ, Cooper C, Carruthers S, Goater M (2006) Persistence with teriparatide in patients with osteoporosis: the UK experience. Osteoporos Int 17:1626–1629. doi:10.1007/s00198-006-0171-5
Grey A, Bolland M, Wattie D, Horne A, Gamble G, Reid IR (2010) Prolonged antiresorptive activity of zoledronate: a randomized, controlled trial. J Bone Miner Res 25:2251–2255. doi:10.1002/jbmr.103
US Food and Drug Administration (2002) Forteo [prescribing information].
Acknowledgements
The authors thank Anna Kaufman, MPH, and Melissa Stauffer, PhD, in collaboration with ScribCo, for medical writing assistance.
This study was funded by Merck & Co., Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Patient data are de-identified, so informed consent and institutional review board approval were not required for this study.
Conflicts of interest
AM, RI, and JW are employees of Merck & Co., Inc. AM owns stock in the company. SS was an employee of Merck & Co., Inc. and owned stock in the company at the time of the study. EML has received consulting and/or speaker honoraria from Merck & Co. Inc., AbbVie, AgNovos Healthcare, Alexion Pharmaceuticals, Amgen, Eli Lilly and Company, Radius Health, Shire, and TheraNova. EML has received research grant support from Merck & Co., Inc., Amgen, and Eli Lilly and Company and serves as a board member for the National Osteoporosis Foundation, the International Society for Clinical Densitometry, and the Osteoporosis Foundation of New Mexico. STH has received consulting honoraria from Merck & Co., Inc., Alexion Pharmaceuticals, Amgen, Eli Lilly and Company, Gilead Sciences, Primus Pharmaceuticals, and Radius Health.
Additional information
S. Sajjan recently deceased.
Rights and permissions
About this article
Cite this article
Modi, A., Sajjan, S., Insinga, R. et al. Frequency of discontinuation of injectable osteoporosis therapies in US patients over 2 years. Osteoporos Int 28, 1355–1363 (2017). https://doi.org/10.1007/s00198-016-3886-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3886-y