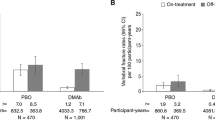
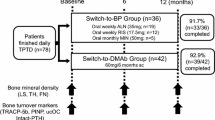
Abstract
Alendronate (ALN) and risedronate (RIS) are ideal as first-choice therapy options in the treatment of postmenopausal osteoporosis. What to do for patients who do not respond adequately to bisphosphonates has not been conclusively determined, but transitioning to other therapies should be considered. The aim of this article is to describe potential alternatives for patients switching from ALN or RIS to other therapies for osteoporosis. A systematic search of PubMed was conducted to find papers that evaluate the effects of switching therapies on fractures, bone mineral density (BMD), or bone turnover markers. Results from 11 studies that prospectively assessed treatment after ALN or RIS in women with postmenopausal osteoporosis were reviewed. All studies are of short duration (all 24 months or less) and assess the topic of transitioning therapy from ALN or RIS. None of the studies had the statistical power to assess fracture-reduction efficacy. Transitioning from ALN to zoledronic acid maintains therapeutic effects for 12 months. Switching to strontium ranelate, denosumab, or teriparatide causes further increases in BMD. Specifically, transitioning to teriparatide could be used for a limited time for select patients but needs to be followed up with anti-resorptive treatment to prevent a loss of the bone gained. There are only few studies—of short duration—that assess the topic of transitioning therapy from ALN or RIS, although this is a very frequent occurrence in clinical practice. This is especially true if the patient has not reached his/her therapy goal. Further long-term studies are needed.


Similar content being viewed by others
References
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795
Siris ES, Boonen S, Mitchell PJ, Bilezikian J, Silverman S (2012) What’s in a name? What constitutes the clinical diagnosis of osteoporosis? Osteoporos Int 23:2093–2097
Cummings SR, Cosman F, Eastell R, Reid IR, Mehta M, Lewiecki EM (2013) Goal-directed treatment of osteoporosis. J Bone Miner Res 28:433–438
Lewiecki EM, Cummings SR, Cosman F (2013) Treat-to-target for osteoporosis: is now the time? J Clin Endocrinol Metab 98:946–953
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25:2359–2381
Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, Cormier C, Breart G, Meunier PJ, Delmas PD (1996) Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res 11:1531–8
Li Z, Chines AA, Meredith MP (2004) Statistical validation of surrogate endpoints: is bone density a valid surrogate for fracture? J Musculoskelet Neuronal Interact 4:64–74
McClung M, Recker R, Miller P, Fiske D, Minkoff J, Kriegman A, Zhou W, Adera M, Davis J (2007) Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone 41:122–128
Michalska D, Stepan JJ, Basson BR, Pavo I (2006) The effect of raloxifene after discontinuation of long-term alendronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab 91:870–877
Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, Siddhanti S, Man HS, San Martin J, Bone HG (2010) Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 25:72–81
Freemantle N, Satram-Hoang S, Tang ET, Kaur P, Macarios D, Siddhanti S, Borenstein J, Kendler DL, Investigators DAPS (2012) Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 23:317–326
Roux C, Hofbauer LC, Ho PR, Wark JD, Zillikens MC, Fahrleitner-Pammer A, Hawkins F, Micaelo M, Minisola S, Papaioannou N, Stone M, Ferreira I, Siddhanti S, Wagman RB, Brown JP (2014) Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomized open-label study. Bone 58:48–54
Recknor C, Czerwinski E, Bone HG, Bonnick SL, Binkley N, Palacios S, Moffett A, Siddhanti S, Ferreira I, Ghelani P, Wagman RB, Hall JW, Bolognese MA, Benhamou CL (2013) Denosumab compared with ibandronate in postmenopausal women previously treated with bisphosphonate therapy: a randomized open-label trial. Obstet Gynecol 121:1291–1299
Middleton ET, Steel SA, Aye M, Doherty SM (2012) The effect of prior bisphosphonate therapy on the subsequent therapeutic effects of strontium ranelate over 2 years. Osteoporos Int 23:295–303
Boonen S, Marin F, Obermayer-Pietsch B, Simoes ME, Barker C, Glass EV, Hadji P, Lyritis G, Oertel H, Nickelsen T, McCloskey EV, Investigators EUROFORS (2008) Effects of previous antiresorptive therapy on the bone mineral density response to two years of teriparatide treatment in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 93:852–860
Ettinger B, San Martin J, Crans G, Pavo I (2004) Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 19:745–751
Miller PD, Delmas PD, Lindsay R, Watts NB, Luckey M, Adachi J, Saag K, Greenspan SL, Seeman E, Boonen S, Meeves S, Lang TF, Bilezikian JP, Open-label Study to Determine How Prior Therapy with Alendronate or Risedronate in Postmenopausal Women with Osteoporosis Influences the Clinical Effectiveness of Teriparatide Investigators (2008) Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab 93:3785–3793
Cosman F, Wermers RA, Recknor C, Mauck KF, Xie L, Glass EV, Krege JH (2009) Effects of teriparatide in postmenopausal women with osteoporosis on prior alendronate or raloxifene: differences between stopping and continuing the antiresorptive agent. J Clin Endocrinol Metab 94:3772–3780
Reynolds K, Viswanathan HN, O’Malley CD, Muntner P, Harrison TN, Cheetham TC, Hsu JW, Gold DT, Silverman S, Grauer A, Morisky DE (2012) Psychometric properties of the osteoporosis-specific Morisky Medication Adherence Scale in postmenopausal women with osteoporosis newly treated with bisphosphonates. Ann Pharmacother 46:659–670
Palacios S, Agodoa I, Bonnick S, Van den Bergh JP, Ferreira I, Ho PR, Brown JP (2015) Treatment satisfaction in postmenopausal women suboptimally adherent to bisphosphonates who transitioned to denosumab compared with risedronate or ibandronate. J Clin Endocrinol Metab 100:E487–E492
Middleton ET, Steel SA, Aye M, Doherty SM (2010) The effect of prior bisphosphonate therapy on the subsequent BMD and bone turnover response to strontium ranelate. J Bone Miner Res 25:455–462
Misof BM, Paschalis EP, Blouin S, Fratzl-Zelman N, Klaushofer K, Roschger P (2010) Effects of 1 year of daily teriparatide treatment on iliacal bone mineralization density distribution (BMDD) in postmenopausal osteoporotic women previously treated with alendronate or risedronate. J Bone Miner Res 25:2297–2303
Cosman F, Keaveny TM, Kopperdahl D, Wermers RA, Wan X, Krohn KD, Krege JH (2013) Hip and spine strength effects of adding versus switching to teriparatide in postmenopausal women with osteoporosis treated with prior alendronate or raloxifene. J Bone Miner Res 28:1328–1336
Delmas PD, Calvo G, Boers M, Abadie E, Avouac B, Kahan A, Kaufman JM, Laslop A, Lekkerkerker JF, Nilsson P, Van Zwieten-Boot B, Kreutz G, Reginster JY, Group for the Respect of Ethics and Excellence in Science (GREES) (2002) The use of placebo-controlled and non-inferiority trials for the evaluation of new drugs in the treatment of postmenopausal osteoporosis. Osteoporos Int 13:1–5
Kanis JA, Alexandre JM, Bone HG, Abadie E, Brasseur D, Chassany O, Durrleman S, Lekkerkerker JF, Caulin F (2003) Study design in osteoporosis: a European perspective. J Bone Miner Res 18:1133–1138
Solomon DH, Avorn J, Katz JN, Finkelstein JS, Arnold M, Polinski JM, Brookhart MA (2005) Compliance with osteoporosis medications. Arch Intern Med 165:2414–2419
Silverman SL, Gold DT (2008) Compliance and persistence with osteoporosis therapies. Curr Rheumatol Rep 10:118–122
Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, Silverman S (2006) Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 81:1013–1022
Caro JJ, Ishak KJ, Huybrechts KF, Raggio G, Naujoks C (2004) The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int 15:1003–1008
Huybrechts KF, Ishak KJ, Caro JJ (2006) Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone 38:922–928
Diez-Perez A, Adachi JD, Agnusdei D, Bilezikian JP, Compston JE, Cummings SR, Eastell R, Eriksen EF, Gonzalez-Macias J, Liberman UA, Wahl DA, Seeman E, Kanis JA, Cooper C, IOF CSA Inadequate Responders Working Group (2012) Treatment failure in osteoporosis. Osteoporos Int 23:2769–2774
Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P (2008) Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. doi:10.1002/14651858.CD001155.pub2
Wells G, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P (2008) Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. doi: 10.1002/14651858.CD004523.pub3
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR, Pivotal Fracture Trial HORIZON (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Chesnut CH III, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, Felsenberg D, Huss H, Gilbride J, Schimmer RC, Delmas PD, Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE) (2004) Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 19:1241–1249
Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 282:637–645
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C, FREEDOM Trial (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
O’Donnell S, Cranney A, Wells GA, Adachi JD, Reginster JY (2006) Strontium ranelate for preventing and treating postmenopausal osteoporosis. Cochrane Database Syst Rev 4:CD005326
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Ensrud KE, Barrett-Connor EL, Schwartz A, Santora AC, Bauer DC, Suryawanshi S, Feldstein A, Haskell WL, Hochberg MC, Torner JC, Lombardi A, Black DM, Fracture Intervention Trial Long-Term Extension Research Group (2004) Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial Long-term Extension. J Bone Miner Res 19:1259–1269
Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR, FLEX Research Group (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296:2927–2938
Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA, Alendronate Phase III Osteoporosis Treatment Study Group (2004) Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350:1189–1199
Abrahamsen B, Grove EL, Vestergaard P (2014) Nationwide registry-based analysis of cardiovascular risk factors and adverse outcomes in patients treated with strontium ranelate. Osteoporos Int 25:757–762
Obermayer-Pietsch BM, Marin F, McCloskey EV, Hadji P, Farrerons J, Boonen S, Audran M, Barker C, Anastasilakis AD, Fraser WD, Nickelsen T, Investigators EUROFORS (2008) Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res 23:1591–1600
Keel C, Kraenzlin ME, Kraenzlin CA, Muller B, Meier C (2010) Impact of bisphosphonate wash-out prior to teriparatide therapy in clinical practice. J Bone Miner Metab 28:68–76
Acknowledgments
PE is an advisory board member with Amgen, MSD, and Eli Lilly and at the speakers bureau with Amgen and Eli Lilly. PV has received unrestricted grants from MSD and Servier and travel grants from Amgen, Eli Lilly, Novartis, Sanofi-Aventis, and Servier.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eiken, P., Vestergaard, P. Treatment of osteoporosis after alendronate or risedronate. Osteoporos Int 27, 1–12 (2016). https://doi.org/10.1007/s00198-015-3334-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3334-4