Abstract
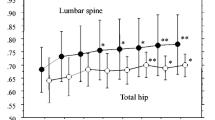
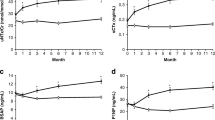
In a 3-year study followed by a 2-year open-label extension, alendronate sodium (ALN) maintained or increased bone mineral density (BMD) in 445 recently postmenopausal women with a spine BMD T-score >−2. In a second 2-year extension, 84 women previously treated with either 5 or 10 mg ALN daily during the first 3 years and 5 mg ALN during the first extension (group A) were randomized to either 5 mg ALN or placebo (PBO). Another group of 59 women (group B) received 20 mg ALN during the first 2 years, PBO during year 3, and were then followed up without treatment during years 4–7. In group A, continuous ALN treatment for 7 years increased spine and trochanter BMD by 2.7–4.1 and 3.3–4.2%, respectively, while femoral neck BMD was maintained. Patients initially receiving 10 mg ALN maintained total body BMD, whereas those treated with 5 mg ALN experienced a small but significant loss after 7 years. Among women who received ALN 5 mg during years 4–7, those who had been treated with ALN 10 mg in the first 3 years had slightly greater increases in BMD at most sites at the end of the study, compared with women who received ALN 5 mg during the first 3 years. During years 6–7, patients who switched to PBO during the previous 2 years showed a significant loss in femoral neck BMD, whereas changes at the other sites were not significant. Women in group B showed significant loss in BMD at all skeletal sites during years 4–7, when they received no treatment. In conclusion, ALN 5 or 10 mg daily for up to 7 years prevents bone loss in recently postmenopausal women. Patients started on ALN 10 mg appear to gain more BMD than those initially treated with 5 mg ALN. Early postmenopausal women who discontinue ALN after 2 years of treatment experience significant bone loss at all skeletal sites despite the higher (20 mg) initial dosing. The ALN was generally well tolerated during 7 years of treatment.




Similar content being viewed by others
References
Riggs BL, Melton LJ (1992) The prevention and treatment of osteoporosis. N Engl J Med 327:620–627
America’s bone health (2002) The state of osteoporosis and low bone mass in our nation. National Osteoporosis Foundation
Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW, Dequeker J, Favus M, Seeman E, Recker RR, Capizzi T, Santora AC, Lombardi A, Shah RV, Hirsch LJ, Karpf DB (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med 333:1437–1443
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of alendronate on risk of fractures in women with existing vertebral fractures. Lancet 348:1535–1541
Karpf DB, Shapiro DR, Seeman E, Ensrud KE, Johnston CC, Adami S, Harris ST, Santora AC, Hirsch LJ, Oppenheimer L, Thompson D (1997) Prevention of nonvertebral fractures by alendronate. J Am Med Assoc 277:1159–1164
Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures. J Am Med Assoc 280:2077–2082
Pols HAP, Felsenberg D, Hanley DA, Stepan J, Munoz-Torres M, Wilkin TJ, Qin-sheng G, Galich AM, Vandormael K, Yates AJ, Stych B (1999) Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Osteoporos Int 9:461–468
Bell NH, Bilezikian JP, Bone HG, Kaur A, Maragoto A, Santora AC (2002) Alendronate increases bone mass and reduces bone markers in postmenopausal African-American women. J Clin Endocrinol Metab 87:2792–2797
Hosking D, Chilvers CED, Christiansen C, Ravn P, Wasnich R, Ross P, McClung M, Baslke A, Thompson D, Daley M, Yates AJ (1998) Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. N Engl J Med 338:485–492
McClung M, Clemmesen B, Daifotis A, Gilchrist NL, Eisman J, Weinstein RS, El Haji Fuleihan G, Reda C, Yates AJ, Ravn P (1998) Alendronate prevents postmenopausal bone loss in women without osteoporosis. Ann Intern Med 128:253–261
Ravn P, Weiss SR, Rodriguez-Portales JA, McClung MR, Wasnich RD, Gilchrist NL, Sambrook P, Fogelman I, Krupa D, AJ Yates, Daifotis A, El-Haji Fuleihan G. Alendronate in early postmenopausal women: effects on bone mass during long-term treatment and after withdrawal
Tonino RP, Meunier PJ, Emkey R, Rodriguez-Portales JA, Menkes CJ, Wasnich RD, Bone HG, Santora AC, Wu M, Desai R, Ross PD (2000) Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. J Clin Endocrinol Metab 85:3109–3115
Writing group for the women’s health initiative investigators (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women. J Am Med Assoc 288:321–333
Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J (2003) Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. J Am Med Assoc 289:2651–2662
Gallagher JC, Rapuri PB, Haynatzki G, Detter JR (2002) Effect of discontinuation of estrogen, calcitriol, and the combination of both on bone density and bone markers. J Clin Endocrinol Metab 87:4914–4923
Greenspan SL, Emkey RD, Bone HG, Weiss SR, Bell NH, Downs RW, McKeever C, Miller SS, Davidson M, Bolognese MA, Mulloy AL, Heyden N, Wu M, Kaur A, Lombardi A (2002) Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. Ann Intern Med 137:875–883
Ascott-Evans BH, Guanabens N, Kivinen S, Stuckey BGA, Magaril CH, Vandormael K, Stych B, Melton ME (2003) Alendronate prevents loss of bone density associated with discontinuation of hormone replacement therapy. Arch Intern Med 163:789–794
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sambrook, P.N., Rodriguez, J.P., Wasnich, R.D. et al. Alendronate in the prevention of osteoporosis: 7-year follow-up. Osteoporos Int 15, 483–488 (2004). https://doi.org/10.1007/s00198-003-1571-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-003-1571-4