Abstract
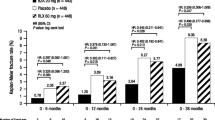
The safety and efficacy of raloxifene, a selective estrogen receptor modulator (SERM), has been studied extensively in large, global clinical trials. However, the effect of raloxifene on bone mineral density (BMD) and on biochemical markers of bone turnover in Japanese postmenopausal women with osteoporosis has not been rigorously evaluated. This study was designed to assess the safety and efficacy of raloxifene in Japanese postmenopausal women with osteoporosis following 1 year of therapy. Participants in this multicenter trial were randomly assigned to receive placebo, raloxifene 60 mg/day (RLX60), or raloxifene 120 mg/day (RLX120). Lumbar spine BMD was measured at baseline, 24, 40, and 52 weeks, and biochemical markers of bone turnover were assessed at baseline, 12, 24, and 52 weeks. Serum lipids were assessed at baseline, 12, 24, 40, and 52 weeks, and breast examinations and transvaginal ultrasound of the endometrium were performed at enrollment and 52 weeks. Compared with baseline, women taking RLX60 had significant increases in lumbar spine (L2-L4) BMD at 24 weeks (+3.3%, p<0.001) through 52 weeks (+3.5%, p<0.001) of therapy, and similar results were observed in the RLX120 group. Markers of bone turnover and total cholesterol and LDL-C were significantly reduced, and no significant treatment-group difference was observed for patients reporting at least one adverse event following randomization. In addition, there were no reported venous thromboembolic events (VTE) in any treatment group. The results of this study demonstrate that raloxifene is associated with early increases in lumbar spine BMD, has favorable effects on biochemical markers of bone turnover and lipid profile, and is well tolerated in postmenopausal Japanese women.




Similar content being viewed by others
References
Ettinger B, Black DM, Mitlak BH et al (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA 282:637–645
Johnston CC Jr, Bjarnason NH, Cohen FJ et al (2000) Long-term effects of raloxifene on bone mineral density, bone turnover, and serum lipipds in early postmenopausal women. 3-year data from two double-blind, randomized, placebo-controlled trials. Arch Intern Med 160:3444–3450
Delmas PD, Ensrud KE, Adachi JD et al (2002) Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab 87:3609–3617
Walsh BW, Kuller LH, Wild RA et al (1998) Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA 279:1445–1451
Barrett-Connor E, Grady D, Sashegyi A et al (2002) Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA 287:847–857
Cummings S, Eckert S, Krueger K et al (1999) The effect of raloxifene on risk of breast cancer in postmenopausal women. JAMA 281:2189–2197
Cauley JA, Norton L, Lippman ME et al (2001) Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Breast Cancer Res Treat 65:125–134
Cohen FJ, Watts S, Shah A et al (2000) Uterine effects of three-year raloxifene therapy in postmenopausal women younger than age 60. Obstet Gynecol 95:104–110
Lufkin EG, Wong M, Deal C (2001) The role of selective estrogen receptor modulators in the prevention and treatment of osteoporosis. Rheum Dis Clin North Am 27:163–185
Maricic M, Adachi JD, Sarkar S et al (2002) Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med 162:1140–1143
Cohen FJ, Lu Y (2000) Characterization of hot flashes reported by healthy postmenopausal women receiving raloxifene or placebo during osteoporosis prevention trials. Maturitas 34:65–73
Kanis JA, Melton LJ3, Christiansen C et al (1994) The diagnosis of osteoporosis. J Bone Miner Res 9:1137–1141
Orimo H et al (1995) The criteria for the diagnosis of primary osteoporosis. The Examination Committee for the Criteria of Osteoporosis Diagnosis—the Japanese Society for Bone and Mineral Research. Osteoporos Int 3:111–116
Lu Y, Ye K, Mathur AK et al (1997) Comparative calibration without a gold standard. Stat Med 16:1889–1905
Lu Y, Mathur AK, Blunt BA et al (1996) Dual X-ray absorptiometry quality control: comparison of visual examination and process-control charts. J Bone Miner Res 11:626–637
Genant HK, Jergas M, Palermo L et al (1996) Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. J Bone Miner Res 11:984–996
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502
Goldstein SR, Scheele WH, Rajagopalan SK et al (2000) A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium . Obstet Gynecol 95:95–103
Brown EG, Wood L, Wood S (1999) The medical dictionary for regulatory activities (MedDRA). Drug Saf 20:109–117
Efron B, Stein C (1981) The jackknife estimate of variance. Ann Stat 9:586–596
Sarkar S, Mitlak BH, Wong M et al (2002) Relationships between bone mineral density and incident vertebral fracture risk with raloxifene therapy. J Bone Miner Res 17:1–10
Delmas PD, Bjarnason NH, Mitlak BH et al (1997) Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med 337:1641–1647
Lips P, Duong T, Oleksik A et al (2001) A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab 86:1212–1221
Acknowledgements
This study was conducted with the support of Eli Lilly Japan KK and Chugai Pharmaceuticals. The authors would like to acknowledge the contributions of Akio Tanaka, Clinical Team Leader for Chugai Pharmaceuticals; Akio Uemura (Eli Lilly Japan KK) and Chuck Rivera (Lilly Research Laboratories) for clinical study design and coordination; Steven Watts (Lilly Research Laboratories) for statistical data analysis; and Timothy Mason and Karen Pinette (Lilly Research Laboratories) for manuscript preparation. The authors would also like to acknowledge the efforts of the the Chief Investigators of the Japan clinical trial: S. Fujimoto, Hokkaido University School of Medicine; F. Komatsuzaki, Sapporo Shirakabadai Hospital; N. Inaba, Dokkyo Medical College; H. Mizunuma, Gunma University School of Medicine; T. Sato, Onarimon Clinic; T. Nitta, Takekawa Hospital; K. Fukuda, Shiratori Clinic; K. Kinoshita, Seijokinoshita Hospital; S. Yamazaki, Sengoku Clinic; A. Itabashi, NS Clinic; K. Nakajima, Ebisu Clinic; K. Suzuki, Suzuki Clinic; S. Yagi, Kamimeguro Internal Medicine Clinic; F. Hirahara, Yokohama City University School of Medicine ; K. Tanaka, Niigata University School of Medicine; K. Inoue, Fujimikogen Hospital; K. Kaneko, Juntendo University Izunagaoka Hospital; N. Hamada, Sumire Hospital; Y. Ohtsuki, Osaka Police Hospital; Y. Kuroki, Kanebo Memorial Hospital; H. Shimada, Kobe City General Hospital; K. Katayama, West Kobe Medical Center; T. Matsuda, Kagoshima Red Cross Hospital; T. Sugimoto, Kobe University School of Medicine; S. Okamoto, Okamoto Internal Medicine Clinic. In addition, recognition is given to the Japan Raloxifene Clinical Team: K. Sato, T. Tominaga, Y. Ishikura, and H. Uchida, Chugai Pharmaceuticals; E. Hamaya, Y. Yoshimoto, J. Nagisa, Y. Hata, T. Yamamoto, and J. Yano, Eli Lilly Japan KK.
Author information
Authors and Affiliations
Additional information
The named authors wrote this article on behalf of the Japan Clinical Trial Research Group.
Rights and permissions
About this article
Cite this article
Morii, H., Ohashi, Y., Taketani, Y. et al. Effect of raloxifene on bone mineral density and biochemical markers of bone turnover in Japanese postmenopausal women with osteoporosis: results from a randomized placebo-controlled trial. Osteoporos Int 14, 793–800 (2003). https://doi.org/10.1007/s00198-003-1424-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-003-1424-1