Abstract
Synthesis of novel thiophene substituted isoxazoline libraries is currently of high interest. Here, we have reported detailed syntheses of 2,5-dichlorothiophene, 5-chloro-2-(benzylthio)thiophene, and 5-chlorothiophene-2-sulphonamide substituted analogs of isoxazoline from chalcones catalyzed by NaOH in PEG-400 as green solvent. In vitro antimycobacterial and antimicrobial activity of newly synthesized compounds were investigated. Compounds 2j, 2k, and 2l showed excellent activity against Mycobacterium tuberculosis and other Mycobacterium strains tested. Compounds 2a–2c, 2i showed excellent activity against all bacterial and fungal species tested. The isoxazoline scaffold synthesized in the present study may be useful in the development of novel new antimicrobial agents.
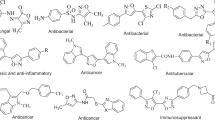
Graphical abstract
An efficient synthesis of isoxazoline libraries of thiophene analogs and its antimycobacterial investigation.



Similar content being viewed by others
References
Acharya AP, Kamble RD, Patil SD, Hese SV, Yemul OS, Patil SG, Hallale SN, Dawane BS (2014) “Green synthesis” of benzothiazepine library of indeno analogues and their in vitro antimicrobial activity. Chem Pap 68(5):719–724
Agarwal H, Kaul N, Paradkar AR, Mahadik KR (2004) HPTLC method for guggulsterone. I. Quantitative determination of E- and Z-guggulsterone in herbal extract and pharmaceutical dosage form. J Pharm Biomed Anal 36:33–41
Ahmadiani A, Javan M, Semnanian S, Barat E, Kamalinejad M (2001) Anti-inflammatory and antipyretic effects of Trigonella foenum-graecum leaves extract. J Ethnopharmacol 75:283–286
Bhowruth V, Dover LG, Besra GS (2007) Tuberculosis chemotherapy: recent developments and future perspectives. Prog Med Chem 45:169–203
Bruhn RD, Madhura BD, Maddox M, Lee RB, Trivedi A, Yang L, Scherman MS, Gilliland GC, Gruppo V, McNeil MR, Lenaerts AJ, Meibohm B, Lee RE (2012) Antitubercular nitrofuran isoxazolines with improved pharmacokinetic properties. Bioorg Med Chem 20(20):6063–6072
Center for Disease Control and Prevention (CDC) (2006) Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drug-worldwide, 2000–2004. MMWR Morb Mortal Wkly Rep 55(11):301–305
Chandrasekhar S, Narsihmulu C, Sultana SS (2002) Poly (ethylene glycol) (PEG) as a reusable solvent medium for organic synthesis. Application in the Heck reaction. Org Lett 4:4399–4401
Chobe SS, Kamble RD, Patil SD, Acharya AP, Hese SV, Yemul OS, Dawane BS (2013) Green approach towards synthesis of substituted pyrazole-1,4-dihydro,9- oxa,1,2,6,8-tetrazacyclopentano[b]naphthalene-5-one derivatives as antimycobacterial agents. Med Chem Res 22:5197–5203
Dawane BS, Konda SG, Mandawad GG, Shaikh BM (2010a) Poly(ethylene glycol) (PEG-400) as an alternative reaction solvent for the synthesis of some new 1-(4-(4′-chlorophenyl)-2-thiazolyl)-3-aryl-5-(2-butyl-4-chloro-1H-imidazol-5yl)-2-pyrazolines and their in vitro antimicrobial evaluation. Euro J Med Chem 45:387–392
Dawane BS, Shaikh BM, Khandare NT, Kamble VT, Chobe SS, Konda SG (2010b) Eco-friendly polyethylene glycol-400: a rapid and efficient recyclable reaction medium for the synthesis of thiazole derivatives. Green Chem Let Rev 3:205–208
Dawane BS, Gacche RN, Yemul OS, Kamble RD, Patil SD, Mogle PP, Hese SV, More RA, Kamble SS, Kote JV (2014) A process for the preparation of thiosemicarbazone encapsulated metal nano particles. Indian Patant Application No. 86/MUM/2014.
Fevig JM, Buriak J, Stouten PFW, Knabb RM, Lam GN, Wong PC, Wexler RR (1999) Preparation of pyrrolidine and isoxazolidine benzamidines as potent inhibitors of coagulation factor Xa. Bioorg Med 9(8):195–1200
Gautam RA, Saklani A, Jachak SM (2007) Indian medicinal plants as a source of antimycobacterial agents. J Ethnopharmacol 110:200–234
Heldebrant D, Jessop PG (2003) Liquid poly (ethylene glycol) and supercritical carbon dioxide: a benign biphasic solvent system for use and recycling of homogeneous catalysts. J Am Chem Soc 125:5600–5601
Jain SL, Singhal S, Sain B (2007) PEG-assisted solvent and catalyst free synthesis of 3,4-dihydropyrimidinones under mild reaction conditions. Green Chem 9:740–741
Kachhadia VV, Patel MR, Joshi HS (2004) Synthesis of isoxazoles and cyanopyridines bearing benzo(b)thiophene nucleus as potential antitubercular and antimicrobial agents. J Sci Islam Repub Iran Winter 15(1):47–51
Kai H, Ichiba T, Tomida M, Masuko M (1999) Synthesis and fungicidal activities of 3-(alpha-alkoxyiminobenzyl)isoxazole derivatives. J Pestic Sci 24:149–155
Kamal A, Bharathi EV, Reddy JS, Janaki M, Ramaiah D, Reddy MK, Viswanath A, Reddy TL, Shaik TB, Pushpavalli SNCVL, Bhadra MP (2011) Synthesis and biological evaluation of 3,5-diaryl isoxazoline/isoxazole linked 2,3-dihydroquinazolinone hybrids as anticancer agents. Eur J Med Chem 46:691–703
Kamble RD, Dawane BS, Yemul OS, Kale AB, Patil SD (2013) Bleaching earth clay (pH 12.5): a green catalyst for rapid synthesis of pyranopyrazole derivatives via a tandem three-component reaction. Res Chem Intermed 39:3859–3866
Kane JL, Hirth BH, Laing O, Gourlie BB, Nahill S, Barsomiam G (2003) Ureas of 5-aminopyrazole and 2-aminothiazole inhibit growth of gram-positive bacteria. Bioorg Med Chem Lett 13:4463–4466
Karabasanagouda T, Adhikari AV, Girisha M (2009) Synthesis of some new pyrazolines and isoxazoles carrying 4-methylthiophenyl moiety as potential analgesic and antiinflamatory agents. Indian J Chem 48B:430–437
Khan KM, Nullah Z, Lodhi MS, Jalil S, Choudhary MI, Rahaman AU (2006) Synthesis and anti-inflammatory activity of some selected aminothiophene analogs. J Enz Inhi Med Chem 21:139–143
Laurenzi M, Ginsberg A, Spigelman M (2007) Challenges associated with current and future TB treatment. Infect Disord Drug Targets 7(2):105–119
Mandawad GG, Chobe SS, Yemul OS, Dawane BS (2011) An efficient green synthesis of some novel hetero chalcones as potent antimicrobial agents. J Pharm Res 4:3360–3363
Meotti FC, Silva DO, dos Santos ARS, Zeni G, Rocha JBT, Nogueira CW (2003) Thiophenes and furans derivatives: a new class of potential pharmacological agents. Env Toxico Pharmco 37:37–44
Nagarajan K, Shankar RG, Rajappa S, Shenoy SJ, Costa-Pereira R (2012) Nitroimidazoles XXI 2,3-dihydro-6-nitroimidazo [2,1-b] oxazoles with antitubercular activity. Euro J Med Chem 47:278–282
National Committee for Clinical Laboratory Standards (1992) Methods for determining bactericidal activity of antimicrobial agents: Tentative Guidelines, Villanova. NCCLS (Publication no NCCLS M 26-T).
National Committee for Clinical Laboratory Standards (1997) Methods for antimicrobial susceptibility testing anaerobic bacteria, Approved Standard-Fourth Edition. Villanova, NCCLS, (Publication no NCCLS M 11-A4).
National Committee for Clinical Laboratory Standards (1998) Performance standards for antimicrobial susceptibility testing. Eighth Information Supplement, Villanova. NCCLS (Publication no NCCLS M 100-58).
Talley JJ, Brown DL, Carter JS, Graneto MJ, Koboldt CM, Masferrer JL, Perkins WE, Rogers RS, Shaffer AF, Zhang YY, Zweifel BS, Seibert K (2000) 4-[5-Methyl-3-phenylisoxazol-4-yl]-benzenesulfonamide, Valdecoxib: a potent and selective inhibitor of COX-2. J Med Chem 43:775–777
Tomioka H (2006) Current status of some antituberculosis drugs and the development of new antituberculous agents with special reference to their invitro and in vivo antimicrobial activities. Curr Pharm Des 12:4047–4070
Velayati AA, Masjedi MR, Farnia P, Tabarsi P, Ghanavi J, Ziazarifi AH, Hoffner SE (2009) Totally drug-resistant TB emerges in India “Nature”. Chest 136:420–425
Walsh C (2003) Where will new antibiotics come from? Nat Rev Microbiol 1:65–70
Acknowledgments
The author RDK is sincerely thankful to the CSIR, New Delhi for JRF. Authors are also thankful to Director Vijayshree Chemicals Hyderabad for gifting 2-n-butyl-4-chloro-5-formyl imidazole (BCFI) and Director, Indian Institute of Chemical Technology Hyderabad, India for providing analytical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mandawad, G.G., Kamble, R.D., Hese, S.V. et al. An efficient synthesis of isoxazoline libraries of thiophene analogs and its antimycobacterial investigation. Med Chem Res 23, 4455–4463 (2014). https://doi.org/10.1007/s00044-014-1016-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1016-y