Abstract
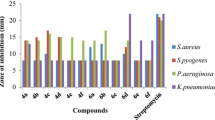
Aluminum trichloride acts as readily available, inexpensive, and efficient catalyst for one-pot three-component condensation reaction of aldehydes, dicarbonyl, and 2-amino benzothiazole under the solvent-free conditions to afford the 4H-pyrimido[2,1-b][1,3]benzothiazole derivatives 4 with good yield. The compounds synthesized in this study were evaluated for their antibacterial activities against gram-positive and gram-negative bacteria, viz., Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella typhi, Escherichia coli, Bacillus cereus, and Providencia rettegeri. Compounds 4c, 4d, 4f, 4g, and 4h showed their good activities against tested bacterial species. Pyrimidine derivatives 4d, 4f, and 4g have shown good antifungal activities against tested fungal strains, such as Aspergillus niger, Aspergillus fumigates, Aspergillus flavus, etc.
Graphical abstract



Similar content being viewed by others
References
Atwal KS, Swanson BN, Unger SE, Floyd DM, Moreland S, Hedberg A, O’reilly BC (1991) Dihydropyrimidine calcium channel blockers. 3. 3-carbamoyl-4-aryl-1, 2, 3,4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid eaters as orally effective antihypertensive agents. J Med Chem 34(2):806–811
Bansal RK (2003) Heterocyclic chemistry, vol 3. New Age International Publishers, New Delhi, p 425
Chitra S, Devanathan D, Pandiarajan K (2010) Synthesis and in vitro microbiological evaluation of novel 4-aryl-5-isopropoxycarbonyl-6-methyl-3,4-dihydropyrimidinones. Eur J Med Chem 45:367–371
Dallinger D, Kappe CO (2007) Microwave-assisted synthesis in water as solvent. Chem Rev 107:2563–2591
Heilmann J, Wasescha MR, Schmidt TJ (2001) The influence of glutathione and cysteine levels on the cytotoxicity of helenanolide type sesquiterpene lactones against KB cells. Bioorg Med Chem 9:2189–2194
Kappe CO (2000) Recent advances in the Biginelli dihydropyrimidine synthesis. New tricks from an old dog. Acc Chem Res 33:879–888
Kappe CO (2002) High-speed combinatorial synthetics utilizing microwave irradiation. Curr Opin Chem Biol 6:314–320
Khoshneviszadeh M, Edraki N, Javidnia K, Alborzi A, Pourabbas B, Mardaneh J, Miri R (2009) Synthesis and biological evaluation of some new 1,4-dihydropyrimidines containing different ester substitute and diethyl carbamoyl group as anti-tuberculosis agents. Bioorg Med Chem 17:1579–1586
Kralisch D, Stark A, Korsten S, Kreisel G, Onodruschka B (2005) Energetic, environmental and economic balances: spice up your ionic liquid research efficiency. Green Chem 7:301–309
Lanjewar KR, Rahatgaonkar AM, Chorghade MS, Saraf BD (2009) Synthesis and antimicrobial activity of 5-(2-aminothiazol-4-yl)-3,4-dihydro-4-phenylpyrimidine-2(1H)-one. Ind J Chem 48B:1732–1737
Matzke M, Stolte S, Thiele K, Juffernholz T, Arning J, Ranke J, Welzbiermann U, Jastorff B (2007) The influence of anion species on the toxicity of 1-alkyl-3-methylimidazolium ionic liquids observed in an (eco) toxicological test battery. Green Chem 9:1198–1207
Miwatashi S, Arikawa Y, Matsumoto T, Uga K, Kanzaki N, Imai YN, Ohkawa S (2008) Synthesis and biological activities of 4-phenyl-5-pyridyl-1, 3-thiazole derivatives as selective adenosine A3 antagonists. Chem Pharm Bull (Tokyo) 56(8):1126–1137
Mosman T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Pham T, Cho C, Yun Y (2010) Environmental fate and toxicity of ionic liquids: a review. Water Res 44:352–372
Ramon DJ, Yus M (2005) Asymmetric multicomponent reaction (AMCRs): the new frontier. Angew Chem Int Ed 44(11):1602–1634
Rovnyak GC, Atwal KS, Hedberg A, Kimball SD, Moreland S, Gougoutas JZ, O’Reillly BC, Schwartz J, Malley MF (1992) Dihydropyrimidine calcium channel blockers. 4. Basic 3-substituted-4-aryl-1,4-dihydropyrimidine-5-carboxylic acid esters. Potent antihypertensive agents. J Med Chem 35(17):3254–3263
Shaabani A, Rahmati A, Naderi S (2005) A novel one-pot three-component reaction: synthesis of triheterocyclic 4H-pyrimido[2, 1-b]benzazoles ring systems. Bioorg Med Chem Lett 15:5553–5557
Singh BK, Mishra M, Saxena N, Yadav GP, Maulik PR, Sahoo MK, Gaur RL, Murthy PK, Tripathi RP (2008) Synthesis of 2-sulfanyl-6-methyl-1,4-dihydropyrimidines as a new class of antifilarial agents. Eur J Med Chem 43:2717–2723
Vicini P, Garonikaki A, Incerti M, Busonera B, Poni G, Cabras CA, La Colla P (2003) Synthesis and biological evaluation of benzo[d]isothiazole, benzothiazole and thiazole Schiff bases. Bioorg Med Chem 11:4785–4789
Zhu J, Bienayme HE (2005) Multicomponent reactions. Wiley-VCH, Wienheim
Acknowledgements
We gratefully acknowledge to Mr. Yogesh Sharma, Parabolic drugs Ltd., Chandigarh and SAIF Punjab University, Chandigarh for spectral analytical data.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sahu, P.K., Sahu, P.K., Lal, J. et al. A facile green synthesis and in vitro antimicrobial activity 4H-pyrimido[2,1-b][1,3]benzothiazole derivatives using aluminum trichloride under solvent free conditions. Med Chem Res 21, 3826–3834 (2012). https://doi.org/10.1007/s00044-011-9908-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9908-6