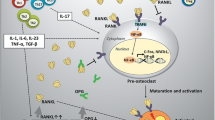
Abstract
Several metabolic, genetic and oncogenic bone diseases are characterized by defective or excessive bone formation. These abnormalities are caused by dysfunctions in the commitment, differentiation or survival of cells of the osteoblast lineage. During the recent years, significant advances have been made in our understanding of the cellular and molecular mechanisms underlying the osteoblast dysfunctions in osteoporosis, skeletal dysplasias and primary bone tumors. This led to suggest novel therapeutic approaches to correct these abnormalities such as the modulation of WNT signaling, the pharmacological modulation of proteasome-mediated protein degradation, the induction of osteoprogenitor cell differentiation, the repression of cancer cell proliferation and the manipulation of epigenetic mechanisms. This article reviews our current understanding of the major cellular and molecular mechanisms inducing osteoblastic cell abnormalities in age-related bone loss, genetic skeletal dysplasias and primary bone tumors, and discusses emerging therapeutic strategies to counteract the osteoblast abnormalities in these disorders of bone formation.


Similar content being viewed by others
References
Seeman E (2002) Pathogenesis of bone fragility in women and men. Lancet 359:1841–1850
Khosla S, Riggs BL (2005) Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin N Am 34:1015–1030
Riggs BL, Parfitt AM (2005) Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res 20:177–184
Kawai M, Modder UI, Khosla S, Rosen CJ (2011) Emerging therapeutic opportunities for skeletal restoration. Nat Rev Drug Discov 10:141–156
Marie PJ, Kassem M (2011) Osteoblasts in osteoporosis: past emerging and future anabolic targets. Eur J Endocrinol 165:1–10
Karsenty G, Kronenberg HM, Settembre C (2009) Genetic control of bone formation. Annu Rev Cell Dev Biol 25:629–648
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192
Long F (2011) Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol 13:27–38
Bianco P, Sacchetti B, Riminucci M (2011) Stem cells in skeletal physiology and endocrine diseases of bone. Endocr Dev 21:91–101
Karsenty G (2001) Minireview: transcriptional control of osteoblast differentiation. Endocrinology 142:2731–2733
Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, Young DW (2006) Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord 7:1–16
Marie PJ (2008) Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys 473:98–105
Bonewald LF (2011) The amazing osteocyte. J Bone Miner Res 26:229–238
Marie PJ (1999) Cellular and molecular alterations of osteoblasts in human disorders of bone formation. Histol Histopathol 14:525–538
Manolagas SC (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137
Marie PJ (1994) Human osteoblastic cells: a potential tool to assess the etiology of pathologic bone formation. J Bone Miner Res 9:1847–1850
Lian JB, Stein GS, van Wijnen AJ, Stein JL, Hassan MQ, Gaur T, Zhang Y (2012) MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol 8:212–227
Bradley EW, McGee-Lawrence ME, Westendorf JJ (2011) Hdac-mediated control of endochondral and intramembranous ossification. Crit Rev Eukaryot Gene Expr 21:101–113
Canalis E (1983) The hormonal and local regulation of bone formation. Endocr Rev 4:62–77
Marie PJ, Jones D, Vico L, Zallone A, Hinsenkamp M, Cancedda R (2000) Osteobiology strain and microgravity: part I. Studies at the cellular level. Calcif Tissue Int 67:2–9
Sims NA, Martin TJ (2014) Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep 3:481
Marie PJ (2009) Bone cell-matrix protein interactions. Osteoporos Int 20:1037–1042
Khosla S (2013) Pathogenesis of age-related bone loss in humans. J Gerontol A Biol Sci Med Sci 38:1228–1235
Manolagas SC, Parfitt AM (2010) What old means to bone. Trends Endocrinol Metab 21:369–374
Ahdjoudj S, Fromigué O, Marie PJ (2004) Plasticity and regulation of human bone marrow stromal osteoprogenitor cells: potential implication in the treatment of age-related bone loss. Histol Histopathol 19:151–157
Kassem M, Marie PJ (2011) Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell 10:191–197
Marie PJ, Kassem M (2011) Extrinsic mechanisms involved in age-related defective bone formation. J Clin Endocrinol Metab 96:600–609
Marie PJ (2014) Bone cell senescence: mechanisms and perspectives. J Bone Miner Res 29:1311–1321
Marie PJ (2001) The molecular genetics of bone formation: implications for therapeutic interventions in bone disorders. Am J Pharmacogenomics 1:175–187
Canalis E (2010) New treatment modalities in osteoporosis. Endocr Pract 16:855–863
Martin TJ (2014) Bone biology and anabolic therapies for bone: current status and future prospects. J Bone Metab 21:8–20
Jilka RL (2007) Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone 40:1434–1446
Compston JE (2007) Skeletal actions of intermittent parathyroid hormone: effects on bone remodelling and structure. Bone 40:1447–1452
Martin TJ (2005) Osteoblast-derived PTHrP is a physiological regulator of bone formation. J Clin Invest 115:2322–2324
Wang H, Liu J, Yin Y, Wu J, Wang Z, Miao D, Sun W (2014) Recombinant human parathyroid hormone related protein 1–34 and 1–84 and their roles in osteoporosis treatment. PLoS One 9:e88237
Horwitz MJ, Augustine M, Khan L, Martin E, Oakley CC, Carneiro RM, Tedesco MB, Laslavic A, Sereika SM, Bisello A, Garcia-Ocana A, Gundberg CM, Cauley JA, Stewart AF (2013) A comparison of parathyroid hormone-related protein (1–36) and parathyroid hormone (1–34) on markers of bone turnover and bone density in postmenopausal women: the PrOP study. J Bone Miner Res 28:2266–2276
Canalis E, Giustina A, Bilezikian JP (2007) Mechanisms of anabolic therapies for osteoporosis. N Engl J Med 357:905–916
Marie PJ (2012) Signaling pathways affecting skeletal health. Curr Osteoporos Rep 10:190–198
Gazzerro E, Canalis E (2006) Bone morphogenetic proteins and their antagonists. Rev Endocr Metab Disord 7:51–65
Simic P, Culej JB, Orlic I, Grgurevic L, Draca N, Spaventi R, Vukicevic S (2006) Systemically administered bone morphogenetic protein-6 restores bone in aged ovariectomized rats by increasing bone formation and suppressing bone resorption. J Biol Chem 281:25509–25521
Fromigué O, Modrowski D, Marie PJ (2004) Growth factors and bone formation in osteoporosis: roles for fibroblast growth factor and transforming growth factor beta. Curr Pharm Des 10:2593–2603
Janssens K, ten Dijke P, Janssens S, Van Hul W (2005) Transforming growth factor-beta1 to the bone. Endocr Rev 26:743–774
Marie PJ (2012) Fibroblast growth factor signaling controlling bone formation: an update. Gene 498:1–4
Kawai M, Rosen CJ (2012) The insulin-like growth factor system in bone: basic and clinical implications. Endocrinol Metab Clin North Am 41:323–333
Engin F, Yao Z, Yang T, Zhou G, Bertin T, Jiang MM, Chen Y, Wang L, Zheng H, Sutton RE, Boyce BF, Lee B (2008) Dimorphic effects of Notch signaling in bone homeostasis. Nat Med 14:299–305
Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R, Long F (2008) Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med 14:306–314
Tao J, Jiang MM, Jiang L, Salvo JS, Zeng HC, Dawson B, Bertin TK, Rao PH, Chen R, Donehower LA, Gannon F, Lee BH (2014) Notch activation as a driver of osteogenic sarcoma. Cancer Cell 26:390–401
Papachroni KK, Karatzas DN, Papavassiliou KA, Basdra EK, Papavassiliou AG (2009) Mechanotransduction in osteoblast regulation and bone disease. Trends Mol Med 15:208–216
Robling AG (2012) The interaction of biological factors with mechanical signals in bone adaptation: recent developments. Curr Osteoporos Rep 10:126–131
Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127:469–480
Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML (2001) LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107:513–523
Baron R, Rawadi G (2007) Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 148:2635–2643
Canalis E (2013) Wnt signaling in osteoporosis: mechanisms and novel therapeutic approaches. Nat Rev Endocrinol 9:575–583
Wagner ER, Zhu G, Zhang BQ, Luo Q, Shi Q, Huang E, Gao Y, Gao JL, Kim SH, Rastegar F, Yang K, He BC, Chen L, Zuo GW, Bi Y, Su Y, Luo J, Luo X, Huang J, Deng ZL, Reid RR, Luu HH, Haydon RC, He TC (2011) The therapeutic potential of the Wnt signaling pathway in bone disorders. Curr Mol Pharmacol 4:14–25
van Bezooijen RL, ten Dijke P, Papapoulos SE, Lowik CW (2005) SOST/sclerostin an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev 16:319–327
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D (2005) Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 280:19883–19887
Paszty C, Turner CH, Robinson MK (2010) Sclerostin: a gem from the genome leads to bone-building antibodies. J Bone Miner Res 25:1897–1904
McClung MR, Grauer A (2014) Romosozumab in postmenopausal women with osteopenia. N Engl J Med 370:1664–1665
Ke HZ, Richards WG, Li X, Ominsky MS (2012) Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 33:747–783
Morvan F, Boulukos K, Clément-Lacroix P, Roman Roman S, Suc-Royer I, Vayssière B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G (2006) Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res 21:934–945
Bodine PV, Stauffer B, Ponce-de-Leon H, Bhat RA, Mangine A, Seestaller-Wehr LM, Moran RA, Billiard J, Fukayama S, Komm BS, Pitts K, Krishnamurthy G, Gopalsamy A, Shi M, Kern JC, Commons TJ, Woodworth RP, Wilson MA, Welmaker GS, Trybulski EJ, Moore WJ (2009) A small molecule inhibitor of the Wnt antagonist secreted frizzled-related protein-1 stimulates bone formation. Bone 44:1063–1068
Zhang J, Tu Q, Bonewald LF, He X, Stein G, Lian J, Chen J (2011) Effects of miR-335-5p in modulating osteogenic differentiation by specifically downregulating Wnt antagonist DKK1. J Bone Miner Res 26:1953–1963
Hassan MQ, Maeda Y, Taipaleenmaki H, Zhang W, Jafferji M, Gordon JA, Li Z, Croce CM, van Wijnen AJ, Stein JL, Stein GS, Lian JB (2012) miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J Biol Chem 287:42084–42092
Guo D, Li Q, Lv Q, Wei Q, Cao S, Gu J (2014) MiR-27a targets sFRP1 in hFOB cells to regulate proliferation apoptosis and differentiation. PLoS One 9:e91354
Haÿ E, Laplantine E, Geoffroy V, Frain M, Kohler T, Müller R, Marie PJ (2009) N-cadherin interacts with axin and LRP5 to negatively regulate Wnt/beta-catenin signaling osteoblast function and bone formation. Mol Cell Biol 29:953–964
Haÿ E, Nouraud A, Marie PJ (2009) N-cadherin negatively regulates osteoblast proliferation and survival by antagonizing Wnt ERK and PI3 K/Akt signaling. PLoS One 4:e8284
Haÿ E, Buczkowski T, Marty C, Da Nascimento S, Sonnet P, Marie PJ (2012) Peptide-based mediated disruption of N-cadherin-LRP5/6 interaction promotes Wnt signaling and bone formation. J Bone Miner Res 27:1852–1863
Haÿ E, Dieudonné FX, Saidak Z, Marty C, Brun J, Da Nascimento S, Sonnet P, Marie PJ (2014) N-Cadherin/Wnt interaction controls bone marrow mesenchymal cell fate and bone mass during aging. J Cell Physiol 229:1765–1775
Revollo L, Kading J, Jeong SY, Li J, Salazar V, Mbalaviele G, Civitelli R (2014) N-cadherin restrains PTH activation of Lrp6/beta-catenin signaling and osteoanabolic action. J Bone Miner Res. doi:10.1002/jbmr2323
Marie PJ, Haÿ E, Saidak Z (2014) Integrin and cadherin signaling in bone: role and potential therapeutic targets. Trends Endocrinol Metab 925:567–575. doi:10.1016/jtem20140600
Gunther T, Poli C, Muller JM, Catala-Lehnen P, Schinke T, Yin N, Vomstein S, Amling M, Schüle R (2005) Fhl2 deficiency results in osteopenia due to decreased activity of osteoblasts. EMBO J 24:3049–3056
Govoni KE, Baylink DJ, Chen J, Mohan S (2006) Disruption of four-and-a-half LIM 2 decreases bone mineral content and bone mineral density in femur and tibia bones of female mice. Calcif Tissue Int 79:112–117
Brun J, Dieudonné FX, Marty C, Muller J, Schüle R, Patino-Garcia A, Lecanda F, Fromigué O, Marie PJ (2013) FHL2 silencing reduces Wnt signaling and osteosarcoma tumorigenesis in vitro and in vivo. PLoS One 8:e55034
Brun J, Fromigué O, Dieudonné FX, Marty C, Chen J, Dahan J, Wei Y, Marie PJ (2013) The LIM-only protein FHL2 controls mesenchymal cell osteogenic differentiation and bone formation through Wnt5a and Wnt10b. Bone 53:6–12
Kansara M, Tsang M, Kodjabachian L, Sims NA, Trivett MK, Ehrich M, Dobrovic A, Slavin J, Choong PF, Simmons PJ, Dawid IB, Thomas DM (2009) Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma and targeted disruption accelerates osteosarcomagenesis in mice. J Clin Invest 119:837–851
Boyce BF, Yao Z, Xing L (2010) Functions of nuclear factor kappaB in bone. Ann N Y Acad Sci 1192:367–375
Krum SA, Chang J, Miranda-Carboni G, Wang CY (2010) Novel functions for NFkappaB: inhibition of bone formation. Nat Rev Rheumatol 6:607–611
Novack DV (2011) Role of NF-kappaB in the skeleton. Cell Res 21:169–182
Chang J, Liu F, Lee M, Wu B, Ting K, Zara JN, Soo C, Al Hezaimi K, Zou W, Chen X, Mooney DJ, Wang CY (2009) NF-kappaB inhibits osteogenic differentiation of mesenchymal stem cells by promoting beta-catenin degradation. Proc Natl Acad Sci USA 110:9469–9474
Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, Guan K, Krebsbach PH, Wang CY (2009) Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med 15:682–689
Yao Z, Li Y, Yin X, Dong Y, Xing L, Boyce BF (2014) NF-kappaB RelB negatively regulates osteoblast differentiation and bone formation. J Bone Miner Res 29:866–877
Yu B, Chang J, Liu Y, Li J, Kevork K, Al-Hezaimi K, Graves DT, Park NH, Wang CY (2014) Wnt4 signaling prevents skeletal aging and inflammation by inhibiting nuclear factor-kappaB. Nat Med 20:1009–1017
Koutsokeras A, Purkayashta N, Rigby A, Subang MC, Sclanders M, Vessillier S, Mullen L, Chernajovsky Y, Gould D (2014) Generation of an efficiently secreted cell penetrating NF-kappaB inhibitor. FASEB J 28:373–381
Herranz D, Serrano M (2010) SIRT1: recent lessons from mouse models. Nat Rev Cancer 10:819–823
Cohen-Kfir E, Artsi H, Levin A, Abramowitz E, Bajayo A, Gurt I, Zhong L, D’Urso A, Toiber D, Mostoslavsky R, Dresner-Pollak R (2011) Sirt1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin a bone formation inhibitor. Endocrinology 152:4514–4524
Simic P, Zainabadi K, Bell E, Sykes DB, Saez B, Lotinun S, Baron R, Scadden D, Schipani E, Guarente L (2013) SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating beta-catenin. EMBO Mol Med 5:430–440
Edwards JR, Perrien DS, Fleming N, Nyman JS, Ono K, Connelly L, Moore MM, Lwin ST, Yull FE, Mundy GR, Elefteriou F (2013) Silent information regulator (Sir)T1 inhibits NF-kappaB signaling to maintain normal skeletal remodeling. J Bone Miner Res 28:960–969
Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M (2010) Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun 1:3
Artsi H, Cohen-Kfir E, Gurt I, Shahar R, Bajayo A, Kalish N, Bellido TM, Gabet Y, Dresner-Pollak R (2014) The sirtuin1 activator SRT3025 down-regulates sclerostin and rescues ovariectomy-induced bone loss and biomechanical deterioration in female mice. Endocrinology 155:3508–3515
Ciechanover A (2005) Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Cell Death Differ 12:1178–1190
Sévère N, Dieudonné FX, Marie PJ (2013) E3 ubiquitin ligase-mediated regulation of bone formation and tumorigenesis. Cell Death Dis 4:e463
Garrett IR, Chen D, Gutierrez G, Zhao M, Escobedo A, Rossini G, Harris SE, Gallwitz W, Kim KB, Hu S, Crews CM, Mundy GR (2003) Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J Clin Invest 111:1771–1782
Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Bonomini S, Crugnola M, Mancini C, Martella E, Ferrari L, Tabilio A, Rizzoli V (2007) The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood 110:334–338
Mukherjee S, Raje N, Schoonmaker JA, Liu JC, Hideshima T, Wein MN, Jones DC, Vallet S, Bouxsein ML, Pozzi S, Chhetri S, Seo YD, Aronson JP, Patel C, Fulciniti M, Purton LE, Glimcher LH, Lian JB, Stein G, Anderson KC, Scadden DT (2008) Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J Clin Invest 118:491–504
Khedgikar V, Kushwaha P, Gautam J, Verma A, Changkija B, Kumar A, Sharma S, Nagar GK, Singh D, Trivedi PK, Sangwan NS, Mishra PR, Trivedi R (2013) Withaferin A: a proteasomal inhibitor promotes healing after injury and exerts anabolic effect on osteoporotic bone. Cell Death Dis 4:e778
Xing L, Zhang M, Chen D (2010) Smurf control in bone cells. J Cell Biochem 110:554–563
Zhang G, Guo B, Wu H, Tang T, Zhang BT, Zheng L, He Y, Yang Z, Pan X, Chow H, To K, Li Y, Li D, Wang X, Wang Y, Lee K, Hou Z, Dong N, Li G, Leung K, Hung L, He F, Zhang L, Qin L (2012) A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nat Med 18:307–314
Jones DC, Wein MN, Oukka M, Hofstaetter JG, Glimcher MJ, Glimcher LH (2006) Regulation of adult bone mass by the zinc finger adapter protein Schnurri-3. Science 312:1223–1227
Glimcher LH, Jones DC, Wein MN (2007) Control of postnatal bone mass by the zinc finger adapter protein Schnurri-3. Ann N Y Acad Sci 1116:174–181
Shu L, Zhang H, Boyce BF, Xing L (2013) Ubiquitin E3 ligase Wwp1 negatively regulates osteoblast function by inhibiting osteoblast differentiation and migration. J Bone Miner Res 28:1925–1935
Tsygankov AY, Teckchandani AM, Feshchenko EA, Swaminathan G (2001) Beyond the RING: CBL proteins as multivalent adapters. Oncogene 20:6382–6402
Salingcarnboriboon RA, Pavasant P, Noda M (2010) Cbl-b enhances Runx2 protein stability and augments osteocalcin promoter activity in osteoblastic cell lines. J Cell Physiol 224:743–747
Sanjay A, Horne WC, Baron R (2001) The Cbl family: ubiquitin ligases regulating signaling by tyrosine kinases. Sci STKE 2001:40
Thien CB, Langdon WY (2005) Negative regulation of PTK signaling by Cbl proteins. Growth Factors 23:161–167
Sévère N, Miraoui H, Marie PJ (2011) The Casitas B lineage lymphoma (Cbl) mutant G306E enhances osteogenic differentiation in human mesenchymal stromal cells in part by decreased Cbl-mediated platelet-derived growth factor receptor alpha and fibroblast growth factor receptor 2 ubiquitination. J Biol Chem 286:24443–24450
Dieudonné FX, Sévère N, Biosse-Duplan M, Weng JJ, Su Y, Marie PJ (2013) Promotion of osteoblast differentiation in mesenchymal cells through Cbl-mediated control of STAT5 activity. Stem Cells 31:1340–1349
Brennan T, Adapala NS, Barbe MF, Yingling V, Sanjay A (2011) Abrogation of Cbl-PI3 K interaction increases bone formation and osteoblast prolifération. Calcif Tissue Int 89:396–410
Aubin JE (2001) Regulation of osteoblast formation and function. Rev Endocr Metab Disord 2:81–94
Bianco P, Robey PG (2001) Stem cells in tissue engineering. Nature 414:118–121
Bianco P, Robey PG, Saggio I, Riminucci M (2010) “Mesenchymal” stem cells in human bone marrow (skeletal stem cells): a critical discussion of their nature identity and significance in incurable skeletal disease. Hum Gene Ther 21:1057–1066
Prockop DJ, Gregory CA, Spees JL (2003) One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci USA 100:11917–11923
Marie PJ, Fromigué O (2006) Osteogenic differentiation of human marrow-derived mesenchymal stem cells. Regen Med 1:539–548
Vilquin JT, Rosset P (2006) Mesenchymal stem cells in bone and cartilage repair: current status. Regen Med 1:589–604
Marie PJ (2013) Targeting integrins to promote bone formation and repair. Nat Rev Endocrinol 9:288–295
Hamidouche Z, Fromigué O, Ringe J, Haüpl T, Vaudin P, Pages JC, Srouji S, Livne E, Marie PJ (2009) Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc Natl Acad Sci USA 106:18587–18591
Kaabeche K, Guénou H, Bouvard D, Didelot N, Listrat A, Marie PJ (2005) Cbl-mediated ubiquitination of alpha5 integrin subunit mediates fibronectin-dependent osteoblast detachment and apoptosis induced by FGFR2 activation. J Cell Sci 118:1223–1232
Srouji S, Ben-David D, Fromigué O, Vaudin P, Kuhn G, Müller R, Livne E, Marie PJ (2012) Lentiviral-mediated integrin alpha5 expression in human adult mesenchymal stromal cells promotes bone repair in mouse cranial and long-bone defects. Hum Gene Ther 23:167–172
Fromigué O, Brun J, Marty C, Da Nascimento S, Sonnet P, Marie PJ (2012) Peptide-based activation of alpha5 integrin for promoting osteogenesis. J Cell Biochem 113:3029–3038
Kumar S, Ponnazhagan S (2007) Bone homing of mesenchymal stem cells by ectopic alpha 4 integrin expression. FASEB J 21:3917–3927
Yao W, Guan M, Jia J, Dai W, Lay YA, Amugongo S, Liu R, Olivos D, Saunders M, Lam KS, Nolta J, Olvera D, Ritchie RO, Lane NE (2013) Reversing bone loss by directing mesenchymal stem cells to bone. Stem Cells 31:2003–2014
Guan M, Yao W, Liu R, Lam KS, Nolta J, Jia J, Panganiban B, Meng L, Zhou P, Shahnazari M, Ritchie RO, Lane NE (2012) Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med 18:456–462
Zhang Y, Wei L, Miron RJ, Shi B, Bian Z (2014) Anabolic bone formation via a site specific bone targeting delivery system by interfering with semaphorin 4d expression. J Bone Miner Res. doi:10.1002/jbmr2322
Wagner EF, Karsenty G (2001) Genetic control of skeletal development. Curr Opin Genet Dev 11:527–532
Ralston SH, Uitterlinden AG (2010) Genetics of osteoporosis. Endocr Rev 31:629–662
Janssens K, Van Hul W (2002) Molecular genetics of too much bone. Hum Mol Genet 11:2385–2393
Shenker A, Chanson P, Weinstein LS, Chi P, Spiegel AM, Lomri A, Marie PJ (1995) Osteoblastic cells derived from isolated lesions of fibrous dysplasia contain activating somatic mutations of the Gs alpha gene. Hum Mol Genet 4:1675–1676
Marie PJ (2001) Cellular and molecular basis of fibrous dysplasia. Histol Histopathol 16:981–988
Riminucci M, Robey PG, Bianco P (2007) The pathology of fibrous dysplasia and the McCune-Albright syndrome. Pediatr Endocrinol Rev 4:401–411
Marie PJ, de Pollak C, Chanson P, Lomri A (1997) Increased proliferation of osteoblastic cells expressing the activating Gs alpha mutation in monostotic and polyostotic fibrous dysplasia. Am J Pathol 150:1059–1069
Riminucci M, Fisher LW, Shenker A, Spiegel AM, Bianco P, Gehron Robey P (1997) Fibrous dysplasia of bone in the McCune-Albright syndrome: abnormalities in bone formation. Am J Pathol 151:1587–1600
Saggio I, Remoli C, Spica E, Cersosimo S, Sacchetti B, Robey PG, Holmbeck K, Cumano A, Boyde A, Bianco P, Riminucci M (2014) Constitutive expression of Gsalpha in mice produces a heritable direct replica of human fibrous dysplasia bone pathology and demonstrates its natural history. J Bone Miner Res. doi:10.1002/jbmr2267
Piersanti S, Remoli C, Saggio I, Funari A, Michienzi S, Sacchetti B, Robey PG, Riminucci M, Bianco P (2010) Transfer analysis and reversion of the fibrous dysplasia cellular phenotype in human skeletal progenitors. J Bone Miner Res 25:1103–1116
Kronenberg HM (2010) Gs signaling in osteoblasts and hematopoietic stem cells. Ann NY Acad Sci 1192:327–329
Sinha P, Aarnisalo P, Chubb R, Ono N, Fulzele K, Selig M, Saeed H, Chen M, Weinstein LS, Divieti Pajevic P, Kronenberg HM, Wu JY (2014) Loss of G alpha early in the osteoblast lineage favors adipogenic differentiation of mesenchymal progenitors and committed osteoblast precursors. J Bone Miner Res. doi:10.1002/jbm2270
Wu JY, Aarnisalo P, Bastepe M, Sinha P, Fulzele K, Selig MK, Chen M, Poulton IJ, Purton LE, Sims NA, Weinstein LS, Kronenberg HM (2011) Gsalpha enhances commitment of mesenchymal progenitors to the osteoblast lineage but restrains osteoblast differentiation in mice. J Clin Invest 121:3492–3504
Regard JB, Cherman N, Palmer D, Kuznetsov SA, Celi FS, Guettier JM, Chen M, Bhattacharyya N, Wess J, Coughlin SR, Weinstein LS, Collins MT, Robey PG, Yang Y (2011) Wnt/beta-catenin signaling is differentially regulated by Galpha proteins and contributes to fibrous dysplasia. Proc Natl Acad Sci USA 108:20101–20106
Ornitz DM, Marie PJ (2002) FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev 16:1446–1465
Lomri A, Lemonnier J, Hott M, de Parseval N, Lajeunie E, Munnich A, Renier D, Marie PJ (1998) Increased calvaria cell differentiation and bone matrix formation induced by fibroblast growth factor receptor 2 mutations in Apert syndrome. J Clin Invest 101:1310–1317
Marie PJ, Kaabeche K, Guénou H (2008) Roles of FGFR2 and twist in human craniosynostosis: insights from genetic mutations in cranial osteoblasts. Front Oral Biol 12:144–159
Dailey L, Ambrosetti D, Mansukhani A, Basilico C (2005) Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev 16:233–247
Suzuki H, Suda N, Shiga M, Kobayashi Y, Nakamura M, Iseki S, Moriyama K (2012) Apert syndrome mutant FGFR2 and its soluble form reciprocally alter osteogenesis of primary calvarial osteoblasts. J Cell Physiol 227:3267–3277
Rice DP, Aberg T, Chan Y, Tang Z, Kettunen PJ, Pakarinen L, Maxson RE, Thesleff I (2000) Integration of FGF and TWIST in calvarial bone and suture development. Development 127:1845–1855
Hajihosseini MK (2008) Fibroblast growth factor signaling in cranial suture development and pathogenesis. Front Oral Biol 12:160–177
Marie PJ, Coffin JD, Hurley MM (2005) FGF and FGFR signaling in chondrodysplasias and craniosynostosis. J Cell Biochem 96:888–896
Mansukhani A, Bellosta P, Sahni M, Basilico C (2000) Signaling by fibroblast growth factors (FGF) and fibroblast growth factor receptor 2 (FGFR2)-activating mutations blocks mineralization and induces apoptosis in osteoblasts. J Cell Biol 149:1297–1308
Mansukhani A, Ambrosetti D, Holmes G, Cornivelli L, Basilico C (2005) Sox2 induction by FGF and FGFR2 activating mutations inhibits Wnt signaling and osteoblast differentiation. J Cell Biol 168:1065–1076
Wilkie AO (2007) Cancer drugs to treat birth defects. Nat Genet 39:1057–1059
Melville H, Wang Y, Taub PJ, Jabs EW (2010) Genetic basis of potential therapeutic strategies for craniosynostosis. Am J Med Genet A 152A:3007–3015
Eswarakumar VP, Ozcan F, Lew ED, Bae JH, Tome F, Booth CJ, Adams DJ, Lax I, Schlessinger J (2006) Attenuation of signaling pathways stimulated by pathologically activated FGF-receptor 2 mutants prevents craniosynostosis. Proc Natl Acad Sci USA 103:18603–18608
Shukla V, Coumoul X, Wang RH, Kim HS, Deng CX (2007) RNA interference and inhibition of MEK-ERK signaling prevent abnormal skeletal phenotypes in a mouse model of craniosynostosis. Nat Genet 39:1145–1150
Miraoui H, Ringe J, Haüpl T, Marie PJ (2010) Increased EGF- and PDGFα-receptor signaling by mutant FGF-receptor 2 contributes to osteoblast dysfunction in Apert craniosynostosis. Hum Mol Genet 19:1678–1689
Moenning A, Jager R, Egert A, Kress W, Wardelmann E, Schorle H (2009) Sustained platelet-derived growth factor receptor alpha signaling in osteoblasts results in craniosynostosis by overactivating the phospholipase C-gamma pathway. Mol Cell Biol 29:881–891
Miraoui H, Marie PJ (2010) Fibroblast growth factor receptor signaling crosstalk in skeletogenesis. Sci Signal 3:re9
Howard TD, Paznekas WA, Green ED, Chiang LC, Ma N, Ortiz de Luna RI, Garcia Delgado C, Gonzalez-Ramos M, Kline AD, Jabs EW (1997) Mutations in TWIST a basic helix-loop-helix transcription factor in Saethre-Chotzen syndrome. Nat Genet 15:36–41
El Ghouzzi V, Legeai-Mallet L, Aresta S, Benoist C, Munnich A, de Gunzburg J, Bonaventure J (2000) Saethre-Chotzen mutations cause TWIST protein degradation or impaired nuclear location. Hum Mol Genet 9:813–819
Bialek P, Kern B, Yang X, Schrock M, Sosic D, Hong N, Wu H, Yu K, Ornitz DM, Olson EN, Justice MJ, Karsenty G (2004) A twist code determines the onset of osteoblast differentiation. Dev Cell 6:423–435
Yousfi M, Lasmoles F, Lomri A, Delannoy P, Marie PJ (2001) Increased bone formation and decreased osteocalcin expression induced by reduced Twist dosage in Saethre-Chotzen syndrome. J Clin Invest 107:1153–1161
Yousfi M, Lasmoles F, Marie PJ (2002) TWIST inactivation reduces CBFA1/RUNX2 expression and DNA binding to the osteocalcin promoter in osteoblasts. Biochem Biophys Res Commun 297:641–644
Miraoui H, Marie PJ (2010) Pivotal role of Twist in skeletal biology and pathology. Gene 468:1–7
Guénou H, Kaabeche K, le Mée SL, Marie PJ (2005) A role for fibroblast growth factor receptor-2 in the altered osteoblast phenotype induced by Twist haploinsufficiency in the Saethre-Chotzen syndrome. Hum Mol Genet 14:1429–1439
Connerney J, Andreeva V, Leshem Y, Mercado MA, Dowell K, Yang X, Lindner V, Friesel RE, Spicer DB (2008) Twist1 homodimers enhance FGF responsiveness of the cranial sutures and promote suture closure. Dev Biol 318:323–334
Miraoui H, Sévère N, Vaudin P, Pagès JC, Marie PJ (2010) Molecular silencing of Twist1 enhances osteogenic differentiation of murine mesenchymal stem cells: implication of FGFR2 signaling. J Cell Biochem 110:1147–1154
Haworth CS, Webb AK, Elkin SL, Hodson ME, Compston JE, Selby PL (2000) Cystic fibrosis related low bone density. Arch Dis Child 83:369
Elkin SL, Vedi S, Bord S, Garrahan NJ, Hodson ME, Compston JE (2002) Histomorphometric analysis of bone biopsies from the iliac crest of adults with cystic fibrosis. Am J Respir Crit Care Med 166:1470–1474
Le Henaff C, Gimenez A, Haÿ E, Marty C, Marie PJ, Jacquot J (2012) The F508del mutation in cystic fibrosis transmembrane conductance regulator gene impacts bone formation. Am J Pathol 180:2068–2075
Le Henaff C, Haÿ E, Velard F, Marty C, Tabary O, Marie PJ, Jacquot JP (2014) Enhanced F508del-CFTR channel activity ameliorates bone pathology in murine cystic fibrosis. Am J Pathol 184:1132–1141
Marie PJ, Fromigué O, Modrowski D (2014) Deregulation of osteoblast differentiation in primary bone cancers. In: Heymann D (ed) Bone cancer: primary bone cancers and bone metastases, 2nd edn. Elseiver, New York
Broadhead ML, Clark JC, Myers DE, Dass CR, Choong PF (2011) The molecular pathogenesis of osteosarcoma: a review. Sarcoma 2011:959248
Thomas DM, Carty SA, Piscopo DM, Lee JS, Wang WF, Forrester WC, Hinds PW (2001) The retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation. Mol Cell 8:303–316
Eferl R, Wagner EF (2003) AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 3:859–868
Dani N, Olivero M, Mareschi K, van Duist MM, Miretti S, Cuvertino S, Patane S, Calogero R, Ferracini R, Scotlandi K, Fagioli F, Di Renzo MF (2012) The MET oncogene transforms human primary bone-derived cells into osteosarcomas by targeting committed osteo-progenitors. J Bone Miner Res 27:1322–1334
Martin JW, Zielenska M, Stein GS, van Wijnen AJ, Squire JA (2011) The role of RUNX2 in osteosarcoma oncogenesis. Sarcoma 2011:282745
Cao Y, Zhou Z, de Crombrugghe B, Nakashima K, Guan H, Duan X, Jia SF, Kleinerman ES (2005) Osterix a transcription factor for osteoblast differentiation mediates antitumor activity in murine osteosarcoma. Cancer Res 65:1124–1128
Basu-Roy U, Seo E, Ramanathapuram L, Rapp TB, Perry JA, Orkin SH, Mansukhani A, Basilico C (2012) Sox2 maintains self renewal of tumor-initiating cells in osteosarcomas. Oncogene 31:2270–2282
Rettew AN, Getty PJ, Greenfield EM (2014) Receptor tyrosine kinases in osteosarcoma: not just the usual suspects. Adv Exp Med Biol 804:47–66
Zhang H, Wu H, Zheng J, Yu P, Xu L, Jiang P, Gao J, Wang H, Zhang Y (2013) Transforming growth factor beta1 signal is crucial for dedifferentiation of cancer cells to cancer stem cells in osteosarcoma. Stem Cells 31:433–446
Rikhof B, de Jong S, Suurmeijer AJ, Meijer C, van der Graaf WT (2009) The insulin-like growth factor system and sarcomas. J Pathol 217:469–482
Lee JA, Ko Y, Kim DH, Lim JS, Kong CB, Cho WH, Jeon DG, Lee SY, Koh JS (2012) Epidermal growth factor receptor: is it a feasible target for the treatment of osteosarcoma? Cancer Res Treat 44:202–209
Sévère N, Dieudonné FX, Marty C, Modrowski D, Patino-Garcia A, Lecanda F, Fromigué O, Marie PJ (2012) Targeting the E3 ubiquitin casitas B-lineage lymphoma decreases osteosarcoma cell growth and survival and reduces tumorigenesis. J Bone Miner Res 27:2108–2117
Hassan SE, Bekarev M, Kim MY, Lin J, Piperdi S, Gorlick R, Geller DS (2012) Cell surface receptor expression patterns in osteosarcoma. Cancer 118:740–749
Jullien N, Dieudonné FX, Habel N, Marty C, Modrowski D, Patino A, Lecanda F, Sévère N, Marie PJ (2013) ErbB3 silencing reduces osteosarcoma cell proliferation and tumor growth in vivo. Gene 521:55–61
Shapovalov Y, Benavidez D, Zuch D, Eliseev RA (2010) Proteasome inhibition with bortezomib suppresses growth and induces apoptosis in osteosarcoma. Int J Cancer 127:67–76
Haydon RC, Deyrup A, Ishikawa A, Heck R, Jiang W, Zhou L, Feng T, King D, Cheng H, Breyer B, Peabody T, Simon MA, Montag AG, He TC (2002) Cytoplasmic and/or nuclear accumulation of the beta-catenin protein is a frequent event in human osteosarcoma. Int J Cancer 102:338–342
Hoang BH, Kubo T, Healey JH, Sowers R, Mazza B, Yang R, Huvos AG, Meyers PA, Gorlick R (2004) Expression of LDL receptor-related protein 5 (LRP5) as a novel marker for disease progression in high-grade osteosarcoma. Int J Cancer 109:106–111
Dieudonné FX, Marion A, Haÿ E, Marie PJ, Modrowski D (2010) High Wnt signaling represses the proapoptotic proteoglycan syndecan-2 in osteosarcoma cells. Cancer Res 70:5399–5408
McQueen P, Ghaffar S, Guo Y, Rubin EM, Zi X, Hoang BH (2011) The Wnt signaling pathway: implications for therapy in osteosarcoma. Expert Rev Anticancer Ther 11:1223–1232
Guo Y, Rubin EM, Xie J, Zi X, Hoang BH (2008) Dominant negative LRP5 decreases tumorigenicity and metastasis of osteosarcoma in an animal model. Clin Orthop Relat Res 466:2039–2045
Dieudonné FX, Marion A, Marie PJ, Modrowski D (2012) Targeted inhibition of T-cell factor activity promotes syndecan-2 expression and sensitization to doxorubicin in osteosarcoma cells and bone tumors in mice. J Bone Miner Res 27:2118–2129
Rao-Bindal K, Kleinerman ES (2011) Epigenetic regulation of apoptosis and cell cycle in osteosarcoma. Sarcoma 2011:679457
Kresse SH, Rydbeck H, Skarn M, Namlos HM, Barragan-Polania AH, Cleton-Jansen AM, Serra M, Liestol K, Hogendoorn PC, Hovig E, Myklebost O, Meza-Zepeda LA (2012) Integrative analysis reveals relationships of genetic and epigenetic alterations in osteosarcoma. PLoS One 7:e48262
Gordon JA, Montecino MA, Aqeilan RI, Stein JL, Stein GS, Lian JB (2014) Epigenetic pathways regulating bone homeostasis: potential targeting for intervention of skeletal disorders. Curr Osteoporos Rep 12:496–506
Cain JE, McCaw A, Jayasekara WS, Rossello FJ, Marini KD, Irving AT, Kansara M, Thomas DM, Ashley DM, Watkins DN (2013) Sustained low-dose treatment with the histone deacetylase inhibitor LBH589 induces terminal differentiation of osteosarcoma cells. Sarcoma 2013:608964
Nugent M (2014) MicroRNA function and dysregulation in bone tumors: the evidence to date. Cancer Manag Res 6:15–25
Miao J, Wu S, Peng Z, Tania M, Zhang C (2013) MicroRNAs in osteosarcoma: diagnostic and therapeutic aspects. Tumour Biol 34:2093–2098
Swami A, Reagan MR, Basto P, Mishima Y, Kamaly N, Glavey S, Zhang S, Moschetta M, Seevaratnam D, Zhang Y, Liu J, Memarzadeh M, Wu J, Manier S, Shi J, Bertrand N, Lu ZN, Nagano K, Baron R, Sacco A, Roccaro AM, Farokhzad OC, Ghobrial IM (2014) Engineered nanomedicine for myeloma and bone microenvironment targeting. Proc Natl Acad Sci USA 111:10287–10292
Magnon C, Lucas D, Frenette PS (2011) Trafficking of stem cells. Methods Mol Biol 750:3–24
Lo Celso C, Lin CP, Scadden DT (2011) In vivo imaging of transplanted hematopoietic stem and progenitor cells in mouse calvarium bone marrow. Nat Protoc 6:1–14
McGee-Lawrence ME, Westendorf JJ (2011) Histone deacetylases in skeletal development and bone mass maintenance. Gene 474:1–11
van Wijnen AJ, van de Peppel J, van Leeuwen JP, Lian JB, Stein GS, Westendorf JJ, Oursler MJ, Im HJ, Taipaleenmaki H, Hesse E, Riester S, Kakar S (2013) MicroRNA functions in osteogenesis and dysfunctions in osteoporosis. Curr Osteoporos Rep 11:72–82
Askmyr M, Sims NA, Martin TJ, Purton LE (2009) What is the true nature of the osteoblastic hematopoietic stem cell niche? Trends Endocrinol Metab 20:303–309
Wu JY, Scadden DT, Kronenberg HM (2009) Role of the osteoblast lineage in the bone marrow hematopoietic niches. J Bone Miner Res 24:759–764
Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, Aung Z, Matza M, Merkenschlager M, Lin C, Rommens JM, Scadden DT (2010) Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 464:852–857
Shiozawa Y, Taichman RS (2012) Getting blood from bone: an emerging understanding of the role that osteoblasts play in regulating hematopoietic stem cells within their niche. Exp Hematol 40:685–694
Kode A, Manavalan JS, Mosialou I, Bhagat G, Rathinam CV, Luo N, Khiabanian H, Lee A, Murty VV, Friedman R, Brum A, Park D, Galili N, Mukherjee S, Teruya-Feldstein J, Raza A, Rabadan R, Berman E, Kousteni S (2014) Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature 506:240–244
Krevvata M, Silva BC, Manavalan JS, Galan-Diez M, Kode A, Matthews BG, Park D, Zhang CA, Galili N, Nickolas TL, Dempster DW, Dougall W, Teruya-Feldstein J, Economides AN, Kalajzic I, Raza A, Berman E, Mukherjee S, Bhagat G, Kousteni S (2014) Inhibition of leukemia cell engraftment and disease progression in mice by osteoblasts. Blood 124:2834–2846
Acknowledgments
The author thanks all members of his group Osteoblast Biology and Pathology who contributed to the work reviewed in this paper, and apologizes to the investigators whose work could not be cited due to space limitations. The author’s work was supported by Centre National de la Recherche Scientifique (CNRS), Institut National de la Recherche Médicale (Inserm), University Paris Diderot, European Commission programs (FP76, FP7), European Calcified Tissue Society (ECTS), Agence Nationale de la Recherche (ANR), Centre National d’Etudes Spatiales (CNES), DIM Stem Pôle Ile de France, Fondation de l’Avenir pour la Recherche Appliquée, Association Recherche contre le Cancer (ARC), Fondation pour la Recherche Médicale (FRM), Société Française de Rhumatologie (SFR), Association Prévention et Traitement des Décalcifications (PTD) and Association Rhumatisme et Travail (Paris, France). Figures were produced using Servier Medical Art.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marie, P.J. Osteoblast dysfunctions in bone diseases: from cellular and molecular mechanisms to therapeutic strategies. Cell. Mol. Life Sci. 72, 1347–1361 (2015). https://doi.org/10.1007/s00018-014-1801-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1801-2