Abstract
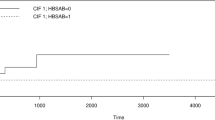
We performed a retrospective survey in 62 hematopoietic cell transplantation (HCT) centers in Japan in which all HCTs performed between 1986 and 1998 were reviewed, and those involving hepatitis B virus surface antigen (HBsAg)-positive donors were identified. One hundred and thirty-five patients who underwent allogeneic HCT (alloHCT) were studied for complications related to hepatitis B virus (HBV) or hepatitis C virus (HCV). The median follow-up period was 24 months. Positivity for HBsAg was observed in 32 patients (24%) throughout the study. Twenty-six of the 32 patients were HBsAg carriers before alloHCT, whereas the remaining 6 became HBsAg(+) after alloHCT. Forty-two recipients were anti-HBs antibody (HBsAb)-positive, and 58 recipients (43%) were HCV Ab(+). Eleven of 26 (42%) HBsAg(+) recipients survived between >4 and >119 months. Six of 26 cases received transplants from HBsAg(+) donors, and, although they had not developed acute graft-versus-host disease, 4 of 6 died of hepatic and renal failure within 10 months after HCT.After transplantation, 5 patients showed serologic evidence of HBV reactivation, whereas 4 patients showed evidence of an immune response to HBV. Viral reactivation occurred during the tapering of the immunosuppressive agent. However, 3 of 5 were alive at the time of this report, suggesting that reactivation is not directly correlated with severe liver dysfunction. Seventeen patients (13%) of 135 recipients developed hepatic failure. Eight (47%) of 17 were diagnosed with fulminant hepatitis and 5 (29%) with veno-occlusive disease (VOD). VOD was observed in 12% of both HBsAg(+) and HCVAb(+) patients. In this study, the relatively high incidence of HBV events occurred after alloHCT, and, therefore, we should consider a protocol for active immunization of donors and recipients against HBV. Moreover, although the presence of HBV or HCV is not a contraindication for alloHCT, we recommend a careful follow-up of recipients after transplantation, especially during immunosuppression tapering.
Similar content being viewed by others
References
Lau GKK, Liang R, Chiu EKW, Lee CK, Lam SK. Hepatic events after bone marrow transplantation in patients with hepatitis B infection: a case controlled study.Bone Marrow Transplant. 1997;19:795–799.
Ustun C, Koc H, Karayalcin S, et al. Hepatitis B virus infection in allogeneic bone marrow transplantation.Bone Marrow Transplant. 1997;20:289–296.
Strasser SI, McDonald GB. Hepatitis viruses and hematopoietic cell transplantation: a guide to patient and donor management.Blood. 1999;93:1127–1136.
Chen YC, Lin KH, Huang WS, Tang JL. Bone marrow transplantation in Taiwan: an overview.Bone Marrow Transplant. 1994;13:705–708.
Dhedin N, Douvin C, Kuentz M, et al. Reverse seroconversion of hepatitis B after allogeneic bone marrow transplantation.Transplantation. 1998;66:616–619.
Frickhofen N, Wiesneth M, Jainta C, et al. Hepatitis C virus infection is a risk factor for liver failure from veno-occlusive disease after bone marrow transplantation.Blood. 1994;83:1998–2004.
Reed EC, Myerson D, Corey L, Meyers JD. Allogeneic marrow transplantation in patients positive for hepatitis B surface antigen.Blood. 1991;77:195–200.
Rodriguez-Inigo E, Thomas JF, Gomez-Garcia de Soria V, et al. Hepatitis C and G virus infection and liver dysfunction after allogeneic bone marrow transplantation: results from a prospective study.Blood. 1997;90:1326–1331.
Carreras E, Bertz H, Arcese W, et al. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European group for blood and marrow transplantation.Blood. 1998;92:3599–3604.
Locasciulli A, Testa M, Valsecchi MG. The role of hepatitis C and B virus infections as risk factors for severe liver complications following allogeneic BMT: a prospective study by the Infectious Disease Working Party of the European Blood and Marrow Transplantation Group.Transplantation. 1999;68:1486–1491.
Locasciulli A, Bacigalupo A, Van Lint MT, et al. Hepatitis B virus (HBV) infection and liver disease after allogeneic bone marrow transplantation: a report of 30 cases.Bone Marrow Transplant. 1990;6:25–29.
Locasciulli A, Alberti A, Banbini G, et al. Allogeneic bone marrow transplantation from HbsAg+ donors: a multicenter study from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO).Blood. 1995;86:3236–3240.
Lau GKK, Lie A, Kwong YL, et al. A case-controlled study on the use of HbsAg(+) donors for allogeneic hematopoietic cell transplantation.Blood. 2000;96:452–458.
Ustun C, Idilman R, Gurman G, et al. Hematopoietic stem cell transplantation from non-replicative hepatitis B virus carriers is safe.J Hepatol. 1999;31:202–209.
Fan FS, Tzeng CH, Yeh HM, et al. Reverse seroconversion of hepatitis B virus infectious status after allogeneic bone marrow transplantation from a carrier donor.Bone Marrow Transplant. 1991;8:417–420.
Webster A, Brenner MK, Prentice HG, et al. Fatal hepatitis B reactivation after autologous bone marrow transplantation.Bone Marrow Transplant. 1989;4:207–213.
Chen PM, Fan S, Liu CJ, et al. Changing of hepatitis B virus markers in patients with bone marrow transplantation.Transplantation. 1990;49:708–713.
Lai CL, Chien RN, Leung N, et al. A one-year trial of lamivudine for chronic hepatitis B.N Engl J Med. 1998;339:61–68.
Dienstag JL, Schiff ER, Wright TL, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States.N Engl J Med. 1999;341:1256–1263.
Locasciulli A, Alberti A. Hepatitis B and hepatitis C virus infections in stem cell transplantation.Leuk Lymphoma. 1999;35:255–260.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hamaguchi, M., Yamad, H., Gondo, H. et al. Retrospective Study on the Impact of Hepatitis B and Hepatitis C Virus Infection on Hematopoietic Stem Cell Transplantation in Japan. Int J Hematol 75, 324–331 (2002). https://doi.org/10.1007/BF02982051
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02982051