Abstract
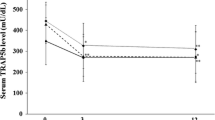
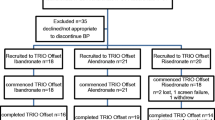
This study was undertaken to compare the effects of alendronate and risedronate on bone mineral density (BMD) and bone turnover markers (BTMs) in late postmenopausal women with osteoporosis. Thirty women older than 60 y of age were randomly assigned to receive alendronate 10 mg (n=16) or risedronate 5 mg (n=14) on a daily basis. The patients were followed every 3 mo for 12 mo. BMD measurements were taken at baseline and at the end of the study, and BTMs were measured at 3-mo intervals. By the end of the study, there were statistically significant increases in BMD in both groups at all sites at which they were measured (P < .001). However, these differences were not statistically significant between groups. By the end of the study, all BTMs had decreased significantly and to a similar extent in both groups. The most significant change was observed in the third month of the study. A negative correlation was noted between percentage change in bonespecific alkaline phosphatase and femoral neck BMD (r=-0.467). This study reported no difference between the 2 drugs in their effects on BMD and BTMs.
Similar content being viewed by others
References
Consensus development conference: diagnosis, prophylaxis and treatment of osteoporosis.Am J Med. 1991; 90: 107–110.
Barrett-Connor E. The economic and human costs of osteoporotic fracture.Am J Med. 1995; 98: 3s-8s.
Garnero P, Delmas PD. Osteoporosis.Endocrinol Metab Clin North Am. 1997; 26: 913–936.
Garnero P, Sornay-Rendu E, Duboeuf MC, Delmas PD. Markers of bone turnover predict postmenopausal forearm bone loss over 4 years: the OFELY study.J Bone Miner Res. 1999; 14: 1614–1621.
Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture risk in elderly women: the EPIDOS prospective study.J Bone Miner Res. 1996; 11: 1531–1538.
Garnero P, Dargent-Molina P, Hans D, et al. Do markers of bone resorption add to bone mineral density and ultrasonographic heel measurement for the prediction of hip fracture in elderly women? The EPIDOS prospective study.Osteoporos Int. 1998; 8: 563–569.
Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group.Lancet. 1996; 348: 1535–1541.
Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial.JAMA. 1998; 280: 2077–2082.
Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group.N Engl J Med. 1995; 333: 1437–1443.
Reginster J, Minne HW, Sorenson OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group.Osteoporos Int. 2000; 11: 83–91.
Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group.JAMA. 1999; 282: 1344–1352.
McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group.N Engl J Med. 2001; 344: 333–340.
Hosking D, Adami S, Felsenberg D, et al. Comparison of change in bone resorption and bone mineral density with once-weekly alendronate and daily risedronate: a randomised, placebocontrolled study.Curr Med Res Opin. 2003; 19: 383–394.
Sarioglu M, Tuzun C, Unlu Z, Tikiz C, Taneli F, Uyanik BS. Comparison of the effects of alendronate and risedronate on bone mineral density and bone turnover markers in postmenopausal osteoporosis.Rheumatol Int. 2006; 26: 195–200.
Sebba AI, Bonnick SL, Kagan R, et al, for the Fosamax Actonel Comparison Trial (FACT) investigators. Response to therapy with once-weekly alendronate 70 mg compared to onceweekly risedronate 35 mg in the treatment of postmenopausal osteoporosis.Curr Med Res Opin. 2004; 20: 2031–2041.
Rosen CJ, Hochberg MC, Bonnick SL, et al, for the Fosamax Actonel Comparison Trial investigators. Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study.J Bone Miner Res. 2005; 20: 141–151.
Mortensen L, Charles P, Bekker PJ, Digennaro J, Johnston CC Jr. Risedronate increases bone mass in early postmenopausal population: two years of treatment plus one year of follow-up.J Clin Endocrinol Metab. 1998; 83: 396–402.
Fogelman I, Ribot C, Smith R, Ethgen D, Sod E, Reginster JY. Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial.J Clin Endocrinol Metab. 2000; 85: 1895–1900.
Kress BC, Mizrahi IA, Armour KW, Marcus R, Emkey RD, Santora AC 2nd. Use of bone alkaline phosphatase to monitor alendronate therapy in individual postmenopausal osteoporotic women.Clin Chem. 1999; 45: 1009–1017.
Chailurkit L, Ongphiphadhanakul B, Piaseu N, Saetung S, Rajatanavin R. Biochemical markers of bone turnover and response of bone mineral density to intervention in early postmenopausal women: an experience in a clinical laboratory.Clin Chem. 2001; 47: 1083–1088.
Yilmaz N, Bayram M, Erbagci AB, Kilincer MS. Diagnostic value of biochemical markers of bone turnover and postmenopausal osteoporosis.Clin Chem Lab Med. 1999; 37: 137–143.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Atmaca, A., Gedik, O. Effects of alendronate and risedronate on bone mineral density and bone turnover markers in late postmenopausal women with osteoporosis. Adv Therapy 23, 842–853 (2006). https://doi.org/10.1007/BF02850205
Issue Date:
DOI: https://doi.org/10.1007/BF02850205