Summary
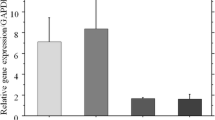
Cultures of osteoblastlike cells obtained from the endosteal surfaces of rabbit long bones formed and mineralized an extracellular matrix when they were supplied daily with medium containing fresh ascorbate. No matrix formed without this supplementation. The matrix mineralized whether or not beta-glycerophosphate, a substrate of alkaline phosphatase, was added to the medium. The ion-transporting ATPase activities of untreated, ascorbate-treated, and ascorbate plus beta-glycerophosphate-treated cells were measured. Ascorbate-treated and ascorbate plus beta-glycerophosphate-treated cells had similar enzyme activities. The activities of the Ca2+-ATPase; Ca2+,Mg2+-ATPase; and alkaline phosphatase in treated cells were elevated over the activities in untreated cells. Na+,K+-ATPase activity was lower in treated than in untreated cells. HCO3 −-ATPase activity was not changed by treatment. Alkaline phosphatase activity was 20 times higher in freshly isolated osteoblastlike cells than in cells grown to confluence in primary culture. In addition, subculturing further reduced the activity of this osteoblast-marker enzyme. The activities of the ion-transporting ATPases and alkaline phosphatase in second passage cells were similar to the activities of these enzymes in fresh, noncalcifying tissues. Nevertheless, second passage cells retain the ability to mineralize an extracellular matrix, and their ion-transporting ATPase and alkaline phosphatase activities are altered when the cells mineralize a matrix.
Similar content being viewed by others
References
Binderman, I.; Duksin, D.; Harell, A.; Katzir, E.; Sachs, L. Formation of bone tissue in culture from isolated bone cells. J. Cell Biol. 61: 427–439; 1974.
Boyum, A. Separation of leukocytes from blodo and bone marrow. Scand. J. Clin. Lab. Invest. 21 (Suppl. 97): 77–89; 1968.
Chow, S. Y.; Kemp, J. W.; Woodbury, D. M. Correlation of iodide transport with Na+,K+-ATPase, HCO3 −-ATPase and carbonic anhydrase activities in different functional states of the rat thyroid gland. J. Endocrinol. 92: 371–379; 1982.
Cohn, D. V.; Wong, G. L. Isolated bone cells. In: Simmons, D. J.; Konin, A. S., eds. Skeletal research, an experimental approach. New York: Academic Press; 1979: 3–20.
Ecarot-Charrier, B.; Glorieur, F. H.; van der Rest M.; Pereira, G. Osteoblasts isolated from mouse calvaria initiate matrix mineralization in culture. J. Cell Biol. 96: 639–643; 1983.
Engstrom, C.; Granstrom, G. Alkaline phosphatases in endochondrial ossification of rats low in calcium and vitamin D deficient. Acta Orthop. Scand. 53: 317–323; 1982.
Farley, J. R.; Ivey, J. L.; Baylink, D. J. Human skeletal alkaline phosphatase. J. Biol. Chem. 255: 4680–4686; 1980.
Felix, R.; Fleisch, H. The pyrophosphatase and (Ca2+-Mg2+)-ATPase activity of purified bone alkaline phosphatase. Biochim. Biophys. Acta 350: 84–94; 1974.
Felix, R.; Fleisch, H. Pyrophosphatase and ATPase of isolated cartilage matrix vesicles. Calcif. Tissue Res. 22: 1–7; 1976.
Harper, J. F.; Brooker, G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2′ 0 acetylation by acetic anhydride in aqueous solution. J. Cyclic Nucleotide Res. 1: 207–218; 1975.
Itaya, K.; Ui, M. A new micromethod for the colorimetric determination of inorganic phosphate. Clin. Chim. Acta 14: 361–366; 1966.
Katz, A. M.; Repke, D. I.; Upshaw, J. E.; Polascik, M. A. Characterization of dog cardiac microsomes. Biochim. Biophys. Acta 205: 473–490; 1970.
Kowarski, S.; Schachter, D. Vitamin D and adenosine triphosphatase dependent on divalent cations in rat intestinal mucosa. J. Clin. Invest. 52: 2765–2773; 1973.
Laemli, U. K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227: 680–685; 1970.
Lieberherr, M.; Vreven, J.; Vaes, G. The acid and alkaline phosphatases, inorganic pyrophosphatases and phosphoprotein phosphatases of bone. Biochim. Biophys. Acta 293: 160–169; 1973.
Lowry, O. H.; Rosebrough, N. J.; Farr, A. L.; Randall, R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275; 1951.
Luben, R. A.; Wong, G. L.; Cohn, D. V. Biochemical characterization with parathormone and calcitonin of isolated bone cells: provisional identification of osteoclasts and osteoblasts. Endocrinology 99: 526–534; 1976.
Majeska, R. J.; Wuthier, R. E. Studies on matrix vesicles isolated from chick epiphyseal cartilage. Association of pyrophosphatase and ATPase activities with alkaline phosphatase. Biochim. Biophys. Acta 391: 51–60; 1975.
Makinose, M. The phosphorylation of the membrane proteins of the sarcoplasmic reticulum during active calcium transport. J. Biochem. 10: 74–82; 1969.
Martin, D. L.; Melancon, M. J; DeLuca, H. F. Vitamin D stmulated, calcium-dependent adenosine triphosphatase from brush borders of rat small intestine. Biochem. Biophys. Res. Commun. 35: 819–823; 1969.
Matthews, J. L.; Vander Wiel, C.; Talmage, R. V. Bone lining cells and the bone fluid compartment, an ultrastructural study. Adv. Exp. Med. Biol. 103: 451–458; 1978.
Melancon, M. J.; DeLuca, H. F. Vitamin D stimulation of calcium-dependent triphosphatase in chick intestinal brush borders. Biochemistry 9: 1658–1664; 1970.
Messer, H. H.; Rogers, J.; Shami, Y.; Copp, D. H. Ca2+, Mg2+-activated ATPase and alkaline phosphatase of developing chick femora. Comp. Biochem. Physiol. (B) 51: 19–24; 1975.
Nijweide, P. J.; van Iperen-van Gent, A. S.; Kawilarang-deHaas, W. W. N.; van der Plas, A. Wassenaar, A. M. Bone formation and calcification by isolated osteoblast-like cells. J. Cell. Biol. 93: 318–323; 1982.
Peck, W. A.; Carpenter, J.; Messinger, K.; DeBra D. Cyclic 3′5′ adenosine monophosphate in isolated bone cells: response to low concentrations of parathyroid hormone. Endocrinology 92: 692–697; 1973.
Posner, A. S. Intramitochondrial storage of stable amorphous calcium phosphate. Ann. NY Acad. Sci. 307: 248–249; 1978.
Prockop, D. J.; Williams, C. J. Structure of the organic matrix: collagen structure (chemical). In: Nancollas, G. H., ed. Biological mineralization and deminseralization. Berlin: Springer-Verlag; 1982: 161–177.
Sandhu, H. S.; Jande, S. S. Investigation of alkaline phosphatase Ca2+-ATPase and Na+,K+-ATPase during beta-APN-induced initial bone mineralization inhibition. Acta Anat. (Basel) 112: 242–248; 1982.
Skillen, A. W.; Rahbani-Nobar, M. Alkaline phosphatase and ATPase acivities of rat bones: separation and characterization. Calcif. Tissue Int. 30: 67–71; 1980.
Spurr, A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. Ultrastruct. Res. 26: 31–43; 1969.
Stekhoven, F. S.; Bonting, S. L. Transport adenosine triphosphatases: properties and functions. Physiol. Rev. 61: 1–76; 1981.
Talmage, R. V. Morphological and physiological considerations in a new concept of calcium transport in bone. Am. J. Anat. 129: 467–476; 1970.
Tenenbaum, H. C. Role of organic phosphate in mineralization of bonein vitro. J. Dent. Res. 60(C): 1586–1589; 1981.
Vaughn, J.The physiology of bone. New York: Oxford University Press; 1981.
Wong, G.; Cohn, D. Separation of parathyroid hormone and calcitonin-sensitive cells from nonresponsive bone cells. Nature 252: 713–715; 1974.
Yee, J. A. Properties of osteoblast-like cells isolated from the cortical endosteal bone surface of adult rabbits. Calcif. Tissue Int. 35: 571–577; 1983.
Author information
Authors and Affiliations
Additional information
This work was supported by Grant NAG-2-108 from the National Aeronautics and Space Administration, Washington, D.C., and Grant 5 PO1 NS15767 from the National Institute of Neurological and Communicative Disorders and Stroke, Bethesda, MD.
Rights and permissions
About this article
Cite this article
Anderson, R.E., Kemp, J.W., Jee, W.S.S. et al. Ion-transporting ATPases and matrix mineralization in cultured osteoblastlike cells. In Vitro 20, 837–846 (1984). https://doi.org/10.1007/BF02619629
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02619629