Summary
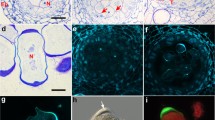
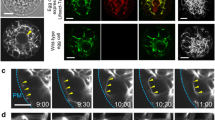
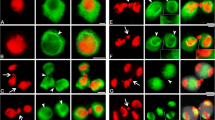
Cytoskeletal organization and chromosome behavior were studied inTradescantia generative cells prior to and during sperm formation using in vitro grown pollen tubes and fluorescence staining methods. Before pollen germination, the crescent-shaped generative cell contains a reticulate microtubule (Mt) system. The cell elongates dramatically after germination, and its Mts assume a helical to longitudinal arrangement. Chromosome condensation is evident approximately 3hr after germination. Kinetochores appear as dark interruptions in the Mt array, and thus seem to attach directly to interphase fibers. No metaphase plate typical of other cells is observed with either DAPI or anti-tubulin staining. Instead, the chromosomes adopt a twisted or braided arrangement, with kinetochores distributed along the length of the cell and kinetochore fibers linked to each other and to surrounding fibers. Anaphase is characterized by a staggered, overlapping separation of chromosomes and by elongation of Mt branches connecting opposing kinetochore fibers. Cytokinesis appears to utilize a furrowing process; a phragmoplast or cell plate was never seen. As a result of these events, the sperm directly inherit their cytoskeleton from generative cell Mts involved in division. No actin fibers are observed at any stage using rhodamine-phalloidin staining. The results are discussed in terms of other reports on sperm formation, possible mitotic and cytokinetic mechanisms, and past distinctions between Mt arrays in higher plant somatic cells.
Similar content being viewed by others
Abbreviations
- CD:
-
cytochalasin D
- DAPI:
-
4′6-diamidino-2-phenyl-indole
- DMSO:
-
dimethylsulfoxide
- K-fiber:
-
kinetochore fiber
- Mf:
-
microfilament
- Mt:
-
microtubule
- PPB:
-
preprophase Mt band
- RP:
-
rhodamine phalloidin
References
Anderson E, Sax K (1936) A cytological monograph of the American species ofTrandescantia. Bot Gaz 97: 433–476
Bailey IW (1920) The cambium and its derivative tissues. III. A reconnaissance of cytological phenomena in the cambium. Am J Bot 7: 417–434
Baird WV, Meagher RB (1987) A complex gene superfamily encodes actin in petunia. EMBO J 6: 3223–3231
Bajer AS, Molé-Bajer J (1986) Reorganization of microtubules in endosperm cells and cell fragments of the higher plantHaemanthus in vivo. J Cell Biol 102: 263–281
Bernatzky R, Tanksley SD (1986) Genetics of actin-related sequences in tomato. Theor Appl Gen 72: 314–321
Bhojwani SS, Bhatnagar SP (1974) The embryology of angiosperms. Advent, New York
Brown RC, Lemmon BE (1988) Preprophasic microtubule systems and development of the mitotic spindle in hornworts (Bryophyta). Protoplasma 143: 11–21
Burgess J (1970) Cell shape and mitotic spindle formation in the generative cell ofEndymion non-scriptus. Planta 95: 72–85
Byers B, Abramson DH (1968) Cytokinesis in Hela: post-telophase delay and microtubule-associated motility. Protoplasma 66: 413–435
Cass DD (1973) An ultrastructural and Nomarski-interference study of the sperms of barley. Can J Bot 51: 601–605
—, Karas I (1975) Development of sperm cells in barley. Can J Bot 53: 1051–1062
Cresti M, Ciampolini F, Kapil RN (1984) Generative cells of some angiosperms with particular emphasis on their microtubules. J Submicrosc Cytol 16: 317–326
— —, Tiezzi A (1986) Ultrastructural studies onNicotiana tabacum pollen tubes grown in different culture medium (preliminary results). Acta Bot Neerl 35: 285–292
—, Lancelle SA, Hepler PK (1987) Structure of the generative cell wall complex after freeze substitution in pollen tubes ofNicotiana andImpatiens. J Cell Sci 88: 373–378
Cyr RJ, Palevitz BA (1989) Microtubule-binding proteins from carrot. I. Initial characterization and microtubule bundling. Planta (in press)
Derksen J, Pierson ES, Traas JA (1985) Microtubules in vegetative and generative cells of pollen tubes. Eur J Cell Biol 38: 142–148
Dickinson HG, Sheldon JM (1984) A radial system of microtubules extending between the nuclear envelope and the plasma membrane during early male haplophase in flowering plants. Planta 161: 86–89
Domozych DS (1987) Cell division inCarteria crucifera (Chlorophyta): the role of the endomembrane system and phycoplast. Protoplasma 136: 170–182
Doonan JH, Cove DJ, Corke FMK, Lloyd CW (1987) Pre-prophase band of microtubules, absent from tip-growing moss filaments, arises in leafy shoots during transition to intercalary growth. Cell Motil Cytoskel 7: 138–153
Dumas C, Knox RB, Gaude T (1985) The spatial association of the sperm cells and vegetative nucleus in the pollen grain ofBrassica. Protoplasma 124: 168–174
Heslop-Harrison J, Heslop-Harrison Y (1984) The disposition of gamete and vegetative-cell nuclei in the extending pollen tubes of a grass species,Alopecurus pratensis L. Acta Bot Neerl 33: 131–134
Hoefert LL (1969) Fine structure of sperm cells in pollen grains ofBeta. Protoplasma 68: 237–240
Hogan CJ (1987) Microtubule pattern during mitosis in two higher plant species. Protoplasma 138: 126–136
Huitorel P, Kirschner M (1988) The polarity and stability of microtubule capture by the kinetochore. J Cell Biol 106: 151–160
Johnston GW (1941) Cytological studies on male gamete formation in certain angiosperms. Am J Bot 28: 306–319
Joshi HC, Yen TJ, Cleveland DW (1987) In vivo coassembly of a divergent ß-tubulin subunit (CB 6) into microtubules of different function. J Cell Biol 105: 2179–2190
Karas I, Cass DD (1976) Ultrastructural aspects of sperm formation in rye: evidence for cell plate involvement in generative cell division. Phytomorphology 26: 36–45
Keith CH, Feramisco JR, Shelanski M (1981) Direct visualization of fluorescein-labeled microtubules in vitro and in microinjected fibroblasts. J Cell Biol 88: 234–240
Lafleur GJ, Gross AE, Mascarenhas JP (1981) Optimization of culture conditions for the formation of sperm cells in pollen tubes ofTradescantia. Gamete Res 4: 35–40
Lancelle SA, Cresti M, Hepler PK (1987) Ultrastructure of the cytoskeleton in freeze-substituted pollen tubes ofNicotiana alata. Protoplasma 140: 141–150
Lewandowska E, Charzynska M (1977)Tradescantia bracteata pollen in vitro: pollen tube development and mitosis. Acta Soc Bot Polon 46: 587–597
Lewis SA, Gu W, Cowan NJ (1987) Free intermingling of mammalian ß-tubulin isotypes among functionally distinct microtubules. Cell 49: 539–548
Lopata MA, Cleveland DW (1987) In vivo microtubules are copolymers of available ß-tubulin isotypes: localization of each of six vertebrate ß-tubulin isotypes using polyclonal antibodies elicited by synthetic peptide antigens. J Cell Biol 105: 1707–1720
Mabuchi I, Okuno M (1977) The effect of myosin antibody on the division of starfish blastomeres. J Cell Biol 74: 251–263
Maheshwari P (1950) An introduction to the embryology of angiosperms. McGraw-Hill, New York
McConchie CA, Hough T, Knox RB (1987) Ultrastructural analyses of the sperm cells of mature pollen of maize,Zea mays. Protoplasma 139: 9–19
McIntosh JR (1987) Progress in research on mitosis. In: Akkas N (ed) Biomechanics of cell division. Plenum, New York, pp 123–143
Mineyuki Y, Marc J, Palevitz BA (1988) Formation of the oblique spindle in dividing guard mother cells ofAllium. Protoplasma 147: 200–203
Mitchison TJ, Kirschner MW (1985) Properties of the kinetochore in vitro. II Microtubule capture and ATP-dependent translocation. J Cell Biol 101: 766–777
Mogensen HL (1986) On the male germ unit in an angiosperm with bicellular pollen,Hippeastrum vitatum. In: Mulcahy DL, Mulcahy GB, Ottaviano E (eds) Biotechnology and ecology of pollen. Springer, New York Berlin Heidelberg, pp 297–305
—, Wagner VT (1987) Associations among components of the male germ unit following in vivo pollination in barley. Protoplasma 138: 161–172
Němec B (1899) Über die karyokinetische Kerntheilung in der Wurzelspitze vonAllim cepa. Jahrb Wiss Bot 33: 313–336
O'Mara J (1933) Division of the generative nucleus in the pollen tube ofLilium. Bot Gaz 94: 567–578
Ôta T (1957) Division of the generative cell in the pollen tube. Cytologia 22: 15–27
Palevitz BA (1987 a) Actin in the preprophase band ofAllium cepa. J Cell Biol 104: 1515–1519
— (1987 b) The accumulation of F-actin during cytokinesis inAllium. Correlation with microtubule distribution and the effects of drugs. Protoplasma 141: 24–32
— (1988 a) Cytochalasin-induced reorganization of actin inAllium root cells. Cell Motil Cytoskel 9: 283–298
— (1988 b) Microtubular fir-trees in mitotic spindles of onion roots. Protoplasma 142: 74–78
—, Cresti M (1988) Microtubule organization in the sperm ofTradescantia virginiana. Protoplasma 146: 28–34
—, Hepler PK (1974) The control of the plane of division during stomatal differentiation inAllium. I. Spindle reorientation. Chromosoma 46: 297–326
Perdue TD, Parthasarathy MV (1985) In situ localization of F-actin in pollen tubes. Eur J Cell Biol 39: 13–20
Pickett-Heaps JD (1974) Plant microtubules. In: Robards AW (ed) Dynamic aspects of plant ultrastructure. McGraw-Hill, New York, pp 219–255
— (1975) Green algae: structure, reproduction and evolution in selected genera. Sinauer Associates, Sunderland, MA
—, Tippet DH, Porter KR (1982) Rethinking mitosis. Cell 29: 729–744
Pierson ES (1988) Rhodamine-phalloidin staining of F-actin in pollen after dimethysulphoxide permeabilization. Sex Plant Reprod 1: 83–87
—, Derksen J, Traas JA (1986) Organization of microfilaments and microtubules in pollen tubes grown in vitro or in vivo in various angiosperms. Eur J Cell Biol 41: 14–18
Raudaskoski M, Anstrom H, Perttila K, Virtanen I, Louhelainen J (1987) Role of the microtubule cytoskeleton in pollen tubes: an immunocytochemical and ultrastructural approach. Biol Cell 61: 177–188
Russell SD (1984) Ultrastructure of the sperm ofPlumbago zeylanica. II. Quantitative cytology and three-dimensional organization. Planta 162: 385–391
— (1985) Preferential fertilization inPlumbago: ultrastructural evidence for gamete-level recognition in an angiosperm. Proc Natl Acad Sci USA 82: 6129–6132
— (1986) Dimorphic sperm, cytoplasmic transmission and preferential fertilization inPlumbago zeylanica. In: Mantell SH, Chapman GP, Street P (eds) The chondriome. Longman, London, pp 69–116
—, Cass DD (1981) Ultrastructure of the sperms ofPlumbago zeylanica. I Cytology and association with the vegetative nucleus. Protoplasma 107: 85–107
Salmon ED, Leslie RJ, Saxton WM, Karow ML, McIntosh JR (1984) Spindle microtubule dynamics in sea urchin embryos: analyses using a fluorescein-labeled tubulin and measurements of fluorescence redistribution after laser photobleaching. J Cell Biol 99: 2165–2174
Sanger JM, Jackson WT (1971 a) Fine structure study of pollen development inHaemanthus katherinae Baker. I. Formation of vegetative and generative cells. J Cell Sci 8: 289–301
— (1971 b) Fine structure study of pollen development inHaemanthus katherinae Baker. II. Microtubules and elongation of the generative cells. J Cell Sci 8: 303–315
Sax K, O'Mara JG (1941) Mechanism of mitosis in pollen tubes. Bot Gaz 102: 629–636
Schibier MJ, Pickett-Heaps JD (1987) The kinetochore fiber structure in the acentric spindles of the green algaOedogonium. Protoplasma 137: 29–44
Schmit A-C, Lambert A-M (1987) Characterization and dynamics of cytoplasmic F-actin in higher plant endosperm cells during interphase, mitosis and cytokinesis. J Cell Biol 105: 2157–2166
—, Vantard M, de Mey J, Lambert A-M (1983) Aster-like microtubule centers establish spindle polarity during interphase-mitosis transition in higher plant cells. Plant Cell Rep 2: 285–288
Schroeder TE (1976) Actin in dividing cells: evidence for its role in cleavage but not mitosis. In: Goldman R, Pollard TD, Rosenbaum J (eds) Cell motility A. Cold Spring Harbor Press, New York, pp 265–277
Segaar PJ, Lokhorst GM (1988) Dynamics of the microtubular cytoskeleton in the green algaAphanochaete magna (Chlorophyta). I. Late mitotic stages and the origin and development of the phycoplast. Protoplasma 142: 176–187
Stanley RG, Linskens HF (1974) Pollen: biology, biochemistry, management. Springer, New York Berlin Heidelberg
Tang X, Hepler PK (1989) Fluorescence microscopic localization of actin in pollen tubes: comparison of actin antibody and phalloidin staining. Cell Motil Cytoskel (in press)
Tiezzi A, Cresti M, Ciampolini F (1986) Microtubules inNicotiana pollen tubes: ultrastructural, immunofluorescence and biochemical data. In: Cresti M, Dallai R (eds) Biology of reproduction and cell motility in plants and animals, University of Siena, Siena, pp 87–94
Wick SM (1985) Immonofluorescence microscopy of tubulin and microtubule arrays in plant cells. III. Transition between mitotic/ cytokinetic and interphase microtubule arrays. Cell Biol Int Rep 9: 357–371
—, Duniec J (1983) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. I Preprophase band development and concomitant appearance of nuclear-envelope-associated tubulin. J Cell Biol 97: 235–243
— — (1984) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. II. Transition between the preprophase band and the mitotic spindle. Protoplasma 122: 45–55
Williamson RE, McCurdy DW, Hurley UA Perkin JL (1987) Actin ofChara giant internode cells. A single isoform in the subcortical filament bundles and a larger immunologically-related protein in the chloroplasts. Plant Physiol 85: 268–272
Witmer SW (1937) Morphology and cytology ofVallisneria spiralis L. Am Nat 18: 309–333
Wylie, RB (1923) Sperm ofVallisneria spiralis. Bot Gaz 75: 191–201
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Palevitz, B.A., Cresti, M. Cytoskeletal changes during generative cell division and sperm formation inTradescantia virginiana . Protoplasma 150, 54–71 (1989). https://doi.org/10.1007/BF01352921
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01352921