Abstract
Segments of mouse parotid were placed in a superfusion chamber. Surface acini were impaled by one or two micro-electrodes for measurement of membrane potential and resistance. The acinus under investigation was stimulated by micro-iontophoretic application of acetylcholine (ACh) or adrenaline.
Neighbouring acinar cells were electrically coupled. Electrical coupling between acinar cells only occurred within restricted domains probably corresponding to an acinus or a group of acini.
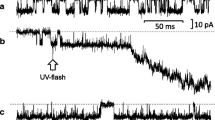
Passing direct current through one intracellular electrode, the resting potential of an acinus could be set at desired levels and the dependency of the ACh-evoked potential change on the resting potential investigated. The ACh null potential (initial effect) was about −60mV. A delayed hyperpolarizing effect of ACh could not be reversed.
The initial ACh-evoked potential change was sensitive to alterations in extracellular Na, K and Cl concentration. The delayed ACh-evoked hyperpolarization was blocked by ouabain, exposure to Na-free or K-free solutions.
It is concluded that ACh increases mainly K and Na membrane conductance causing K efflux and Na influx with a subsequent Na activation of an electrogenic Na pump.
Similar content being viewed by others
References
Ginsborg, B. L.: Electrical changes in the membrane in junctional transmission. Biochim. Biophys. Acta300, 289–317 (1973)
Ginsborg, B. L., House, C. R., Silinsky, E. M.: Conductance changes associated with the secretory potential in the cockroach salivary gland. J. Physiol. (Lond.)236, 723–731 (1974)
Imai, Y.: Study of the secretion mechanism of the submaxillary gland of dog. Part 2. Effect of changing ions in the perfusate on salivary secretion and secretory potential, with special reference to the ionic distribution in the gland tissue. J. Physiol. Soc. Jap.27, 313–324 (1965)
Imai, Y.: On secretory processes and membrane potential of dog submaxillary gland. In: Secretory mechanims of exocrine glands (N. A. Thorn and O. H. Petersen, eds.), p. 251. Copenhagen: Munksgaard 1974
Iwatsuki, N., Petersen, O. H.: Pancreatic acinar cells: localization of acetylcholine receptors and the importance of chloride and calcium for acetylcholine-evoked depolarization. J. Physiol. (Lond.)269, 723–733 (1977a)
Iwatsuki, N., Petersen, O. H.: Pancreatic acinar cells: the acetylcholine equilibrium potential and its ionic dependency. J. Physiol. (Lond.)269, 735–751 (1977b)
Iwatsuki, N., Petersen, O. H.: Pancreatic acinar cells: acetylcholine-evoked electrical uncoupling and its ionic dependency. J. Physiol. (Lond.)274, 81–96 (1978a)
Iwatsuki, N., Petersen, O. H.: Membrane potential, resistance, and intercellular communication in the lacrimal gland: effects of acetylcholine and adrenaline. J. Physiol. (Lond.)275, 507–520 (1978b)
Kagayama, M., Nishiyama, A.: Membrane potential and input resistance in acinar cells from cat and rabbit submaxillary glands in vivo: effects of autonomic nerve stimulation. J. Physiol. (Lond.)242, 157–172 (1974)
Koelz, H. R., Kondon, S., Blum, A. L., Schulz, I.: Calcium ion uptake induced by cholinergic and α-adrenergic stimulation in isolated cells of rat salivary glands. Pflügers Arch.370, 37–44 (1977)
Lundberg, A.: The mechanism of establishment of secretory potentials in sublingual gland cells. Acta Physiol. Scand.40, 35–58 (1957)
Nishiyama, A., Petersen, O. H.: Pancreatic acinar cells: membrane potential and resistance change evoked by acetylcholine. J. Physiol. (Lond.)238, 145–158 (1974a)
Nishiyama, A., Petersen, O. H.: Membrane potential and resistance measurements in acinar cells from salivary glands in vitro: effect of acetylcholine. J. Physiol. (Lond.)242, 173–188 (1974b)
Nishiyama, A., Petersen, O. H.: Pancreatic acinar cells: ionic dependence of acetylcholine-induced membrane potential and resistance change. J. Physiol. (Lond.)244, 431–465 (1975)
Pedersen, G. L., Petersen, O. H.: Membrane potential measurement in parotid acinar cells. J. Physiol. (Lond.)234, 217–227 (1973)
Petersen, O. H.: Some factors influencing stimulation-induced release of potassium from the cat submandibular gland to fluid perfused through the gland. J. Physiol. (Lond.)208, 431–447 (1970a)
Petersen, O. H.: The dependence of the transmembrane salivary secretory potential on the external potassium and sodium concentration. J. Physiol. (Lond.)210, 205–215 (1970b)
Petersen, O. H.: The ionic transports involved in the acetylcholine-induced change in membrane potential in acinar cells from salivary glands and their importance in the salivary secretion process. In: Electrophysiology of epithelial cells (G. Giebisch, ed.), pp. 207–221. Stuttgart: Schattauer 1971
Petersen, O. H.: Membrane potential measurement in mouse salivary gland cells. Experientia29, 160–161 (1973a)
Petersen, O. H.: Electrogenic sodium pump in pancreatic acinar cells. Proc. R. Soc. London [Biol.]184, 115–119 (1973b)
Petersen, O. H., Pedersen, G. L.: Membrane effects mediated by alpha- and beta-adrenoceptors in mouse parotid acinar cells. J. Membr. Biol.16, 353–362 (1974)
Petersen, O. H., Ueda, N.: Pancreatic acinar cells: the role of calcium in stimulus-secretion coupling. J. Physiol. (Lond.)254, 583–606 (1976)
Petersen, O. H., Ueda, N., Hall, R. A., Gray, T.: The role of calcium in parotid amylase secretion evoked by excitation of cholinergic, α- and β-adrenergic receptors. Pflügers Arch.372, 231–237 (1977)
Poulsen, J. H.: Acetylcholine induced transport of Na+ and K+ in the perfused cat submandibular gland. Pflügers Arch.349, 215–220 (1974)
Poulsen, J. H., Bledsoe, S. W.: Salivary gland K+ transport: in vivo study with K+-specific microelectrodes. Am. J. Physiol.234, E79-E83 (1978)
Roberts, M. L., Petersen, O. H.: Membrane potential and resistance changes induced in salivary gland acinar cells by microiontophoretic application of acetylcholine and adrenergic agonists. J. Membr. Biol.39, 297–312 (1978)
Schneyer, L. H., Schneyer, C. A.: Electrolyte and inulin spaces of rat salivary glands and pancreas. Am. J. Physiol.199, 649–652 (1960)
Skou, J. C.: Enzymatic basis for active transport of Na and K across cell membrane. Physiol Rev.45, 596–619 (1965)
Trautwein, W., Dudel, J.: Zum Mechanismus der Membranwirkung des Acetylcholin an der Herzmuskelfaser. Pflügers Arch.266, 324–334 (1958)
Yoshimura, H., Imai, Y.: Studies on the secretory potential of acinar cell of dog's submaxillary gland and the ionic dependency of it. Jpn. J. Physiol.17, 280–293 (1967)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Roberts, M.L., Iwatsuki, N. & Petersen, O.H. Parotid acinar cells: ionic dependence of acetylcholine-evoked membrane potential changes. Pflugers Arch. 376, 159–167 (1978). https://doi.org/10.1007/BF00581579
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00581579