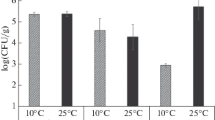
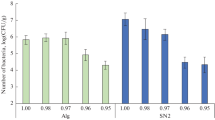
Summary
Approximately 1500 cultures of microorganisms were isolated from rocks and soils of the Ross Desert (McMurdo-Dry Valleys). From these, 15 coccoid strains were chosen for more detailed investigation. They were characterized by morphological, physiological and chemotaxonomical properties. All isolates were Grampositive, catalase-positive and nonmotile. Six strains showed red pigmentation and could be identified as members of the genera Micrococcus (M. roseus, M. agilis) or Deinococcus. In spite of their coccoid morphology, the remaining nine strains had to be associated with coryneform bacteria (Arthrobacter, Brevibacterium), because of their cell wall composition and G+C ratios. Most of the strains were psychrotrophic, but one strain was even obligately psychrophilic, with a temperature maximum below 20°C. Red cocci had in vitro pH optima above 9.0 although they generally originated from acid samples. Most isolates showed a preference for sugar alcohols and organic acids, compounds which are commonly known to be released by lichens, molds and algae, the other components of the cryptoendolithic ecosystem. These properties indicate that our strains are autochthonous members of the natural Antarctic microbial population.
Similar content being viewed by others
References
Anderson DG, McKay LL (1983) Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol 46:549–552
Baird-Parker AC (1965) The classification of Staphylococci and Micrococci from world-wide sources. J Gen Microbiol 38:363–387
Boyd WL, Staley JT, Boyd JW (1966) Ecology of soil microorganisms of Antarctica. In: Tedrow ICF (ed) Antarctic soils and soil forming processes, vol 8. Antarct Res Ser. Am Geophys Union, Washington, pp 125–159
Brooks BW, Murray RGE (1981) Nomenclature for “Micrococcus radiodurans” and other radiation-resistent cocci: Deinococcaceae fam. nov. and Deinococcus gen. nov., including five species. Int J Syst Bacteriol 31:353–360
Brooks BW, Murray RGE, Johnson JL, Stackebrandt E, Woese CR, Fox GE (1980) Red-pigmented micrococci: A basis of taxonomy. Int J Syst Bacteriol 30:627–646
Cooney JJ, Marks HW, Smith AM (1966) Isolation and identification of canthaxanthin from Micrococcus roseus. J Bacteriol 92:342–345
Counsell TJ, Murray RGE (1986) Polar lipid profiles of the genus Deinococcus. Int J Syst Bacteriol 36:202–206
Craigie JS (1974) Storage products. In: Stewart WDP (ed) Algal physiology and biochemistry, vol 10. Blackwell, London, pp 206–235
Darling CA, Siple PA (1941) Bacteria of Antarctica. J Bacteriol 42:83–98
Eckardt FEW, Roggentin P, Hirsch P (1979) Fatty acid composition of various hyphal budding bacteria. Arch Microbiol 120:81–85
Friedmann EI (1982) Endolithic microorganisms in the Antarctic cold desert. Science 215:1045–1053
Friedmann EI, Ocampo R (1976) Endolithic blue-green algae in the dry valleys: primary producers and the Antarctic desert ecosystem. Science 193:1247–1249
Friedmann EI, Garty J, Kappen L (1980) Fertile stages of cryptoendolithic lichens in the dry valleys of southern Victoria Land. Antarct J US 15:166–167
Goodfellow M, Minnikin DE (1981) Introduction to the coryneform bacteria. In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG (eds) The Prokaryotes, vol II. Springer, Berlin Heidelberg New York, pp 1811–1826
Hale ME, Ocampo-Friedmann R (1984) Ascospore cultures of lichen phycobionts from the Antarctic desert. Antarct J US 19:170
Harper JJ, Davis GHG (1979) Two-dimensional thinlayer chromatography for amino acid analysis of bacterial cell walls. Int J Syst Bacteriol 29:56–58
Hellebust JA (1974) Extracellular products. In: Stewart WDP (ed) Algal physiology and biochemistry, vol 10. Blackwell, London, pp 838–863
Hill DJ (1970) The carbohydrate movement between the symbionts of lichens. D Phil thesis, University of Oxford
Hirsch P, Gallikowski C, Friedmann E (1985) Microorganisms in soil samples from Linneaus Terrace southern Victoria Land: Preliminary observations. Antarct J US 19:183–186
Ishida Y, Kadota H (1981) Growth patterns and substrate requirements of naturally occurring obligate oligotrophs. Microbiol Ecol 7:123–130
Johnson RM, Bellinoff RD (1981) Characteristics of cold desert Antarctic coryneform bacteria. In: Parker E (ed) Terrestrial biology, III. Antarct Res Ser, vol 30, pp 169–184
Johnson RM, Inai M, McCarthy S (1981) Characteristics of cold desert Antarctic coryneform bacteria. J Arizona-Nevada Acad Sci 16:51–60
Johnson RM, Madden JM, Swaford JR (1978) Taxonomy of Antarctic bacteria from soils and air primarily of the McMurdo station and Victoria Land dry valleys region. In: Parker E (ed) Terrestrial biology, III. Antarctic Res Ser, vol 30, pp 35–64
Jones D, Collins MD (1986) Irregular, nonsporing gram-positive rods. In: Krieg NR (ed) Bergey's manual of systematic bacteriology, vol 2, 9th edn. Williams and Wilkins, Baltimore, pp 1261–1434
Kappen L (1983) Ecology and physiology of the Antarctic fructicose lichen Usnea sulphurea (Koenig) Th. Fries. Polar Biol 1:249–255
Kappen L, Friedmann EI (1983) Ecophysiology of lichens in the dry valleys of southern Victoria Land, Antarctica. 2 CO2 gas exchange in cryptoendolithic lichens. Polar Biol 1:227–232
Keddie RM, Jones D (1981) Saprophytic, aerobic coryneform bacteria. In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG (eds) The Prokaryotes, vol II. Springer, Berlin Heidelberg New York, pp 1838–1878
Lewis DH, Smith DC (1967) Sugar alcohols (polyols) in fungi and green plants. I. Distribution, physiology and metabolism. New Phytol 66:143–184
MacKay MW, Al-Bakri GH, Moseley BEB (1985) The plasmids of Deinococcus spp. and the cloning and restriction mapping of the D. radiophilus plasmid pUE1. Arch Microbiol 141:91–94
Madden JM, Siegel SK, Johnson RM (1978) Taxonomy of some Antarctic Bacillus and Corynebacterium species. In: Parker E (ed) Terrestrial biology, III. Antarct Res Ser, vol 30, pp 77–103
Mandel M, Igambi L, Bergendahl J, Donson ML, Scheltgen E (1970) Correlation of melting temperature and caesium chloride buoyant density of bacterial desoxyribonucleic acid. J Bacteriol 101:333–338
Marmur J (1961) A procedure for the isolation of desoxyribonucleic acid from microorganisms. J Mol Biol 3:208–218
Marmur J, Doty P (1962) Determination of the base composition of desoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol 5:109–118
Mathis JN, Kloos WE (1984) Isolation and characterization of Micrococcus plasmids. Curr Microbiol 10:339–344
McLean AL (1918) Bacteria of ice and snow in Antarctica. Nature 102:35–39
Morita RY (1975) Psychrophilic bacteria. Bacteriol Rev 39:144–167
Richardson DHS, Hill DH, Smith DC (1968) Lichen physiology. XI. The role of the alga in determining the pattern of carbohydrate movement between lichen symbionts. New Phytol 67:469–486
Schleifer KH (1986) Gram-positive cocci. In: Krieg NR (ed) Bergey's manual of systematic bacteriology, 9th edn, vol 2. Williams and Wilkins, Baltimore, pp 999–1103
Schleifer KH, Kandler O (1972) Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev 36:407–477
Smibert RM, Krieg NR (1981) General characterization. In: Manual of methods for general microbiology. American Soc Microbiol Washington DC, pp 409–433
Stackebrandt E, Lewis BJ, Woese CR (1980) The phylogenetic structure of the coryneform group of bacteria. Zentralbl Bakteriol Mikrobiol Hyg Abt II Orig C 1:137–149
Staley JT (1968) Prosthecomicrobium and Ancalomicrobium, new prostecate fresh water bacteria. J Bacteriol 95:1922
Stokes JL (1963) Recent progress in microbiology. Gibbons NE (ed). University of Toronto Press, Toronto, pp 187
Straka RP, Stokes JL (1960) Psychrophilic bacteria from Antarctica. J Bacteriol 80:622–625
Tearle PV (1987) Cryptogamic carbohydrate release and microbial response during spring freeze-thaw cycles in Antarctic fellfield fines. Soil Biol Biochem 19:381–389
Vestal JR, Friedmann EI (1982) In situ carbon metabolism by the cryptoendolithic microbial community in the Antarctic cold desert. Antarct J US 17:190–191
Vishniac HS (1982) An enation system for the isolation of Antarctic yeasts inhibited by conventional media. Can J Microbiol 29:90–95
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Siebert, J., Hirsch, P. Characterization of 15 selected coccal bacteria isolated from antarctic rock and soil samples from the McMurdo-Dry Valleys (South-Victoria Land). Polar Biol 9, 37–44 (1988). https://doi.org/10.1007/BF00441762
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00441762