Abstract
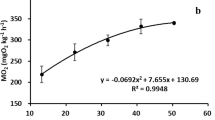
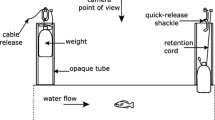
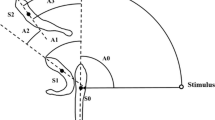
Escape-swimming speeds (U max) were studied in settled turbot (Scophthalmus maximus L.) reared at 18°C. Metamorphosis was complete at 4.0 cm total length (TL). U max scaled in proportion to TL0.74 in fish of 0.88 5o 8.00 cm TL at 18▿C. The scaling relationship for U max was similar for temperatures between 13 and 23°C and could be fitted by the model: \(U_{\max } = 28.4 + 10.9\left( {\frac{{temp - 13}}{5}} \right) + 10.3{\text{ }}TL\). U max temperature-dependent, with a Q10 of 1.77 over the temperature range studied. Analysis of covariance showed that U max for farmed turbot was 14% lower than for wild fish filmed within 2 wk of capture; 3 mo after capture the average differences in escape performance were no longer significant, which suggests that the lower escape speeds of farmed fish are due to acclimation effects and not genetic stock differences. In order to assess the individual variability of U max, 18 wild juvenile turbot [TL=6.2±0.4 cm (Week 1) to 7.5±0.5 cm (Week 17); means±SD] were maintained in individual containers at 18°C. U max was determined weekly for 6 wk, standardised for fish length using the scaling relationship U max=1.46 TL 0.74, and individuals were ranked in order of performance. Temperature was reduced after 6 wk to 13°C, resulting in a significant decline in U max from 104.0±14.4 to 87.8±12.5 cm s-1 (means±SD). After 3 wk at 13°C U max had increased to a level not significantly different from that at 18°C. Kendall's coefficient of concordance showed that repeatability of ranking of the experimental U max of individuals was maintained over a 13 wk period and through temperature change. The results demonstrate that escape-swimming speeds in juvenile turbot are repeatable, individually variable, and can be modified in response to temperature acclination.
Similar content being viewed by others
References
Al-Maghazachi SJ, Gibson R (1984) The development stages of larval turbot, Scophthalmus maximus (L.). J exp Biol 82: 35–51
Archer SD, Johnston JA (1989) Kinematics of labriform and subcarangiform swimming in the Antarctic fish Notothenia neglecta. J exp Biol 143: 195–210
Bailey KM, Batty RS (1984) Laboratory study of predation by Aurelia aurita on larvae of cod, flounder, plaice and herring: development and vulnerability to capture. Mar Biol 83: 287–291
Batty RS (1984) Development of swimming movements and musculature of larval herring (Clupea harengus). J exp Biol 110: 217–229
Batty RS, Blaxter JHS (1992) The effect of temperature on the burst swimming performance of fish larvae. J exp Biol 170: 187–201
Batty RS, Blaxter JHS, Bone Q (1991) The effect of temperature on the swimming of a teleost (Clupea harengus) and an ascidian larva (Dendrodoa grossularia). Comp Biochem Physiol 100A: 297–300
Batty RS, Blaxter JHS, Fretwell K (1993) Effect of temperature on the escape responses of larval herring, Clupea harengus. Mar Biol 115: 523–528
Beddow TA, Johnston IA (1995) Plasticity of muscle contractile properties following temperature acclimation in the marine fish, Myoxocephalus scorpius (in preparation)
Beddow TA, Van Leeuwen JL, Johnston IA (1995) Swimming kinematics of fast starts are altered by temperature acclimation in the marine fish, Myoxocephalus scorpius. J exp Biol 196: (in press)
Bennett AF (1987) Interindividual variability: an underutilized resource. In: Feder ME, Bennet AF, Burggrer WW, Huey B (eds) New directions in ecological physiology. Cambridge University Press, New York, pp 147–169
Brooks S, Johnston IA (1993) Influence of development and rearing temperature on the distribution, ultrastructure and myosin subunit composition of myotomal muscle-fibre types in the plaice Pleuronectes platessa. Mar Biol 117: 501–513
Calvo J, Johnston IA (1992) Influence of rearing temperature on the distribution of muscle fibre types in the turbot Scophthalmus maximus at metamorphosis. J exp mar Biol Ecol 161: 45–55
Crockford T, Johnston IA (1993) Developmental changes in the composition of myofibrillar proteins in the swimming muscles of Atlantic herring, Glupea harengus. Mar Biol 115: 15–22
Day F (1880–1884) The fishes of Great Britain and Ireland. Vol. II. Williams & Norgate, London
Edwards AL (1962) Statistical methods for the behavioural sciences. Holt, Rinehart & Winston, New York
El-Fiky N, Hinterleitner S, Wieser W (1987) Differentiation of swimming muscles and gills and development of anaerobic power in the larvae of cyprinid fish (Pisces, Teleostei). Zoomorphology 107: 126–132
Fuiman LA (1986) Burst swimming performance of larval zebra danios and the effects of diel temperature fluctuations. Trans Am Fish Soc 115: 143–148
Gibson RN, Ansell AD, Robb L (1993) Seasonal and annual variations in abundance and species composition of fish and macrocrustacean communities on a Scottish sandy beach. Mar Ecol Prog Ser 98: 89–105
Guderley H, Blier P (1988) Thermal acclimation in fish: conservative and labile properties of swimming muscle. Can J Zool 66: 1105–1115
Jayne BC, Bennett AF (1993) Selection on locomotor performance capacity in a natural population of garter snakes. Evolution 44: 1204–1229
Johnston IA (1993) Phenotypic plasticity of fish muscle to temperature change. In: Rankin JC, Jensen FB (eds) Fish ecophysiology. Chapman & Hall, London, pp 322–340
Johnston IA, Davison W, Gldspink G (1977) Energy metabolism of carp swimming muscles. J comp Physiol 114: 203–216
Johnston IA, Horne Z (1994) Immunocytochemical investigations of muscle differentiation in the Atlantic herring (Clupea harengus: Teleostei). J mar biol Ass UK 74: 79–91
Johnston IA, Lucking M (1978) Temperature induced variation in the distribution of different types of muscle fibres in the goldfish (Carassius carssius). J comp Physiol 124: 111–116
Johnston IA, Sidell BD, Driedzic WR (1985) Force-velocity characteristics and metabolism of carp muscle fibres following temperature acclimation. J exp Biol 119: 239–249
Kolok AS (1992a) Short communication: the swimming performances of individual largemouth bass (Micropterus salmoides) are repeatable. J exp Biol 170: 265–270
Kolok AS (1992b) Morphological and physiological correlates with swimming performance in juvenile largemouth bass. Am J Physiol (Regul integrat comp Physiol 32) 263: R1042-R1048
Kruuk H (1963) Diurnal periodicity in the activity of the common sole Solea vulgaris quensel. Neth J Sea Res 2: 1–28
Langfeld KS, Crockford T, Johnston JA (1991) Temperature acclimation in the common carp: force-velocity characteristics and myosin sub-unit composition of slow muscle fibres. J exp Biol 155: 291–304
Pearson MP, Spriet LL, Stevens ED (1990) Effect of spring training on swim performance and white muscle metabolism during exercise and recovery in rainbow trout (Salmo gairdneri). J exp Biol 149: 45–60
Precht H (1958) Concepts of the temperature adaptation of unchanging reaction systems of cold-blooded animals. In: Prosser CL (ed) Physiological adaption, Washington DC, American ociation for the Advancement of Science, pp 50–78
Scapolo PA, Veggetti A, Mascarello F, Romanello MF, (1988) Developmental transitions of myosin isoforms and organisation of the lateral muscle in the teleost Dicentrarchus labrax (L.). Anat Embryol 178: 287–296
Sidell BD (1980) Response of goldfish (Carassius auratus L.) muscle to acclimation temperature: alterations in biochemistry and proportions of different fibre types. Pyhsiol, Zoöl 53: 93–107
Sisson JE, Sidell BD (1987) Effect of thermal acclimation on muscle fibre recruitment of swimming stripped bass. Physiol, Zoöl 63: 310–320
Sokal RR, Rohlf FJ (1981) Biometry. The principles and practice of statistics in biological research. 2nd edn. W. H. Freeman & Co, New York
Taylor EB, McPhail JD (1985a) Variation in burst and prolonged swimming performance among British Columbia populations of coho salmon, Oncorhynchus kisutch. Can J Fish aquat Sciences 42: 2029–2033
Taylor EB, McPhail JD (1985b) Prolonged and burst swimming in anadromous and freshwater threespine stickleback, Gasterosteus aculeatus. Can J Zool 64: 416–420
Van Der Veer HW, Bergman MJN (1987) Predation by crustaceans on a newly settled 0-group plaice Pleuronectes platessa population in the Wadden Sea. Mar Ecol Prog Ser 31: 121–129
Vieira VLA, Johnston IA (1992) Influence of temperature on muscle-fibre development in larvae of the herring Clupea harengus. Mar Biol 112: 333–341
Waller U (1992) Factors influencing routine oxygen consumption in turbot, Scophthalmus maximus. J appl Ichthyol 8: 62–71
Webb PW (1976) Effects of median-fin amputation on fast-start performance of rainbow trout, Salmo gairdneri. J exp Biol 68: 123–135
Webb PW (1978) Temperature effects on acceleration of rainbow trout, Salmo gairdneri. J Fish Res Bd Can 35: 1417–1422
Williams PJ, Brown JA (1992) Development changes in the escape response of larval winter flounder Pleuronectes americanus from hatch through metamorphosis. Mar Ecol Prog Ser 88: 185–193
Author information
Authors and Affiliations
Additional information
Communicated by J. Mauchline, Oban
Rights and permissions
About this article
Cite this article
Gibson, S., Johnston, I.A. Scaling relationships, individual variation and the influence of temperature on maximum swimming speed in early settled stages of the turbot Scophthalmus maximus . Marine Biology 121, 401–408 (1995). https://doi.org/10.1007/BF00349449
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00349449