Abstract
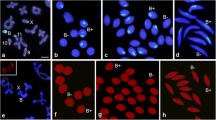
A conspicuous mass of extrachromosomal DNA (Giardina's body) is found in oogonia and oocytes of Dytiscid water beetles. Since in older oocytes this DNA is associated with numerous nucleoli, it seemed probable that the ovary might contain extra copies of the genes for ribosomal RNA (rRNA). This hypothesis has been confirmed by centrifugation and molecular hybridization studies. —In Dytiscus marginalis and Colymbetes fuscus a high density satellite DNA is found in somatic cells and in sperm. Hybridization experiments show that all of the rDNA, i.e., those sequences complementary to rRNA, are located in this satellite, although quantitatively they make up only a small fraction of the satellite. In both species the DNA isolated from ovariole tips is enriched with respect to the satellite. A parallel enrichment of the rDNA has been shown in ovariole tips of Colymbetes, but for technical reasons has not been examined in Dytiscus. —The following model is proposed. In somatic cells and sperm the rDNA is part of an extensive region of high density DNA in one or more chromosomes. In oogonia and oocytes the entire high density region is replicated extrachromosomally and appears cytologically as Giardina's body.
Similar content being viewed by others
References
Bauer, H.: Die wachsenden Oocytenkerne einiger Insekten in ihrem Verhalten zur Nuklealfärbung. Z. Zellforsch. 18, 254–298 (1933).
Bayreuther, K.: Die Oogenese der Tipuliden. Chromosoma (Berl.) 7, 508–557 (1956).
Bier, K., W. Kunz u. D. Ribbert: Struktur und Funktion der Oocytenchromosomen und Nukleolen sowie der Extra-DNS während der Oogenese panoistischer und meroistischer Insekten. Chromosoma (Berl.) 23, 214–254 (1967).
Birnstiel, M. L., H. Wallace, J. L. Sirlin, and M. Fischberg: Localization of the ribosomal DNA complements in the nucleolar organizer region of Xenopus laevis. Nat. Cancer Inst. Monogr. 23, 431–447 (1966).
Brachet, J.: La localisation de l'acide thymonucléique pendant l'oogénèse et la maturation chez les amphibiens. Arch. Biol. (Liège) 51, 151–165 (1940).
Britten, R. J., and D. E. Kohne: Repeated sequences in DNA. Science 161, 529–540 (1968).
Brown, D. D., and I. B. Dawid: Specific gene amplification in oocytes. Science 160, 272–280 (1968).
—, C. S. Weber, and J. H. Sinclair: Ribosomal RNA and its genes during oogenesis and development. Carnegie Inst. Wash. Year Book 66, 580–589 (1967).
Buchner, P.: Das accessorische Chromosom in Spermatogenese und Oogenese der Orthopteren, zugleich ein Beitrag zur Kenntnis der Reduktion. Arch. Zellforsch. 3, 335–430 (1909).
Debaisieux, P.: Les débuts de l'ovogénèse dans le Dytiscus marginalis. La Cellule 25, 207–236 (1909).
Ebstein, B. S.: Tritiated actinomycin D as a cytochemical label for small amounts of DNA. J. Cell Biol. 35, 709–713 (1967).
Evans, D., and M. Birnstiel: Localization of amplified ribosomal DNA in the oocyte of Xenopus laevis. Biochim. biophys. Acta (Amst.) 166, 274–276 (1968).
Ficq, A., and E. Urbani: Cytochemical studies on the oogenesis of “Dytiscus marginalis L.” (In press.)
Flamm, W. G., H. E. Bond, and H. E. Burr: Density-gradient centrifugation of DNA in a fixed-angle rotor. Biochim. biophys. Acta (Amst.) 129, 310–317 (1966).
—, M. Mccallum, and P. M. B. Walker: The isolation of complementary strands from a mouse DNA fraction. Proc. nat. Acad. Sci. (Wash.) 57, 1729–1734 (1967).
Gall, J. G.: Differential synthesis of the genes for ribosomal RNA during amphibian oogenesis. Proc. nat. Acad. Sci. (Wash.) 60, 553–560 (1968).
Giardina, A.: Origine dell'oocite e della cellule nutrici nel Dytiscus. Int. Mschr. Anat. Physiol. 18, 418–484 (1901).
Gillespie, D., and S. Spiegelman: A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J. molec. Biol. 12, 829–842 (1965).
Günthert, T.: Die Eibildung der Dytisciden. Zool. Jb. Abt. Anat. u. Ontog. 30, 301–372 (1910).
Guyénot, E., et M. Danon: Chromosomes et ovocytes de Batraciens. Rev. suisse Zool. 60, 1–129 (1953).
Heinonen, L., and O. Halkka: Early stages of oogenesis and metabolic DNA in the oocytes of the house cricket, Acheta domesticus (L.). Ann. Med. exp. Fenn. 45, 101–109 (1967).
Johnson, M. W.: A study of the nucleoli of certain insects and the crayfish. J. Morph. 62, 113–139 (1938).
Kezer, J.: Cited in W. J. Peacock, Chromosome replication. Nat. Cancer Inst. Monogr. 18, 101–131 (1965).
Kidston, M. E.: Nucleolar DNA in oocytes and nurse cells of Dytiscus marginalis. B. Sc. Honours thesis, University of St. Andrews, Scotland, 1968 (unpublished).
King, H. D.: The oogenesis of Bufo lentiginosus. J. Morph. 19, 369–438 (1908).
Kit, S.: Equilibrium sedimentation in density gradients of DNA preparations from animal tissues. J. molec. Biol. 3, 711–716 (1961).
Kunz, W.: Funktionsstrukturen im Oocytenkern von Locusta migratoria. Chromosoma (Berl.) 20, 332–370 (1967a).
—: Lampenbürstenchromosomen und multile Nukleolen bei Orthopteren. Chromosoma (Berl.) 21, 446–462 (1967b).
Kunz, W.: Die Entstehung multipler Oocytennukleolen aus akzessorischen DNS-Körpern bei Gryllus domesticus. Chromosoma (Berl.) 26, 41–75 (1969).
Lima-de-faria, A.: Metabolic DNA in Tipula oleracea. Chromosoma (Berl.) 13, 47–59 (1962).
— and M. J. Moses: Ultrastructure and cytochemistry of metabolic DNA in Tipula. J. Cell Biol. 30, 177–192 (1966).
— B. Nilsson, D. Cave, A. Puga, and H. Jaworska: Tritium Labelling and Cytochemistry of Extra DNA in Acheta. Chromosoma (Berl.) 25, 1–20 (1968).
Macgregor, H. C.: Nucleolar DNA in oocytes of Xenopus laevis. J. Cell Sci. 3, 437–444 (1968).
Miller, O. L.: Extrachromosomal nucleolar DNA in amphibian oocytes. J. Cell Biol. 23, 60A (1964).
—: Structure and composition of peripheral nucleoli of salamander oocytes. Nat. Cancer Inst. Monogr. 23, 53–66 (1966).
Nilsson, B.: DNA-bodies in the germline of Acheta domesticus (Orthoptera). Hereditas (Lund) 56, 396–398 (1966).
Painter, T. S., and A. N. Taylor: Nucleic acid storage in the toad's egg. Proc. nat. Acad. Sci. (Wash.) 28, 311–317 (1942).
Perkowska, E., H. C. Macgregor, and M. L. Birnstiel: Gene amplification in the oocyte nucleus of mutant and wild-type Xenopus laevis. Nature (Lond.) 217, 649–650 (1968).
Ritossa, F. M., K. C. Atwood, D. L. Lindsley, and S. Spiegelman: On the chromosomal distribution of DNA complementary to ribosomal and soluble RNA. Nat. Cancer Inst. Monogr. 23, 449–472 (1966).
—, and S. Spiegelman: Localization of DNA complementary to ribosomal RNA in the nucleolus organizer region of Drosophila melanogaster. Proc. nat. Acad. Sci. (Wash.) 53, 737–745 (1965).
Urbani, E., e S. Russo-Caia: Osservazioni citochimiche e autoradiografiche sul metabolismo degli acidi nucleici nella oogenesi di “Dytiscus marginalis” L. Rend. Ist. Sci. Univ. Camerino 5, 19–50 (1964).
Wallace, H., and M. L. Birnstiel: Ribosomal cistrons and the nucleolar organizer. Biochim. biophys. Acta (Amst.) 114, 296–310 (1966).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gall, J.G., Macgregor, H.C. & Kidston, M.E. Gene amplification in the oocytes of dytiscid water beetles. Chromosoma 26, 169–187 (1969). https://doi.org/10.1007/BF00326453
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00326453