Abstract
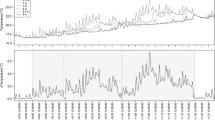
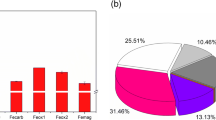
The geochemical response of sediments to increased nutrient input to an Alaskan, arctic lake was examined using direct measurements of sediment-water chemical fluxes. An unexpected increase in Fe flux occurred when sediments were exposed to high incident radiation and nutrient concentrations. Correlation between light and acid-soluble Fe concentrations suggests that photoreduction of Fe(III) oxides may occur, but nutrient addition enhanced the effect indicating that primary productivity was also important. The processes controlling the flux of Fe from sediments in this lake were complex and included the redox potential (dissolved oxygen concentration) of the water, quality of organic matter present in the sediment, light, and nutrients supplied from the sediments and/or water column. These four factors together with the possibility of direct uptake of Fe by phytoplankton and the possible release of algal reductants may contribute to Fe cycling in this lake.
Similar content being viewed by others
References
Behra, P. & L. Sigg, 1990. Evidence for redox cycling of iron in atmospheric water droplets. Nature 344: 419–421.
Collienne, R. H., 1983. Photoreduction of iron in the epilimnion of acidic lakes. Limnol. Oceanogr. 28: 83–100.
Cornwell, J. C., 1983. The geochemistry of manganese, iron and phosphorus in an Alaskan arctic lake. Ph.D. dissertation, Univ. of Alaska Fairbanks, 234 p.
Cornwell, J. C., 1986. Diagenetic trace-metal profiles in arctic lake sediments. Envir. Sci. Technol. 20: 299–302.
Cornwell, J. C., 1987. Phosphorus cycling in arctic lake sediment: adsorption and authigenic minerals. Arch. Hydrobiol. 109: 161–179.
Cotner, J. B. & R. T. Heath, 1990. Iron redox effects of photosensitive phosphorus release from dissolved humic materials. Limnol. Oceanogr. 35: 1175–1181.
Francis, A. J. & C. J. Dodge, 1990. Anaerobic microbial remobilization of toxic metals coprecipitated with iron oxide. Envir. Sci. Technol. 24: 373–378.
Francko, D. A., 1986. Epilimnetic phosphorus cycling: influence of humic materials and iron on coexisting major mechanisms. Can. J. Fish. aquat. Sci. 43: 302–310.
Fuller, C. C. & J. A. Davis, 1989. Influence of coupling of sorption and photosynthetic processes on trace element cycles in natural waters. Nature 340: 52–54.
Gibbs, M. M., 1979. A simple method for the rapid determination of iron in natural waters. Wat. Res. 13: 295–297.
Madsen, E. L., M. D. Morgan & R. E. Good, 1986. Simultaneous photoreduction and microbial oxidation of iron in a stream in the New Jersey Pinelands. Limnol. Oceanogr. 31: 832–838.
McKnight, D. & K. E. Bencala, 1988. Diel variations in iron chemistry in an acidic stream in the Colorado Rocky Mountains, U.S.A. Arctic Alpine Res. 20: 492–500.
McKnight, D. M., B. A. Kimball & K. E. Bencala, 1988. Iron photoreduction and oxidation in an acidic mountain stream. Science 240: 637–640.
Stookey, L. L., 1970. Ferrozine — a new spectrophotometric reagent for iron. Analyt. Chem. 42: 779–298.
Sulzberger, B., D. Suter, C. Siffert, S. Banwart & W. Stumm, 1989. Dissolution of Fe(III) (hydr)oxides in natural waters; laboratory assessment on the kinetics controlled by surface coordination. Mar. Chem. 28: 127–144.
Waite, T. D. & F. M. M. Morel, 1984. Photoreductive dissolution of colloidal iron oxides in natural waters. Envir. Sci. Technol. 18: 860–868.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sugai, S.F., Kipphut, G.W. The influence of light and nutrient addition upon the sediment chemistry of iron in an arctic lake. Hydrobiologia 240, 91–101 (1992). https://doi.org/10.1007/BF00013455
Issue Date:
DOI: https://doi.org/10.1007/BF00013455