Abstract
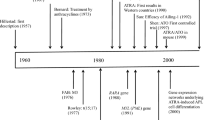
Acute promyelocytic leukemia(APL) is characterized by the t(15;17) chromosomal translocation leading to the formation of the PML-RARα oncoprotein. This leukemia has attracted considerable interest in recent years, being the first in which therapies that specifically target the underlying molecular lesion, i.e., all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), leading to induction of differentiation and apoptosis have been successfully used in clinical practice. The advent of ATRA therapy has transformed APL from being a disease with a poor outlook to one of the most prognostically favorable subsets of acute myeloid leukemia. Further improvements in outcome may be achieved with the use of ATO, which achieves high rates of remission in the relatively small proportion of patients now relapsing following standard first-line therapy with ATRA and anthracycline-based chemotherapy. Moreover, recent studies have suggested that ATO and ATRA, or even ATO alone, used as front-line treatment of PML-RARA- associated APL can induce long-term remissions. This raises the possibility that some patients can be cured using differentiation therapies alone, without the need for chemotherapy, thereby potentially reducing treatment-related toxicity. It is clear that the success of such an approach is critically dependent upon molecular diagnostics and monitoring for minimal residual disease (MRD) to distinguish those patients who can potentially be cured with differentiation therapy from those requiring additional myelosuppressive agents. This represents an exciting new phase in the treatment of acute leukemia, highlighting the potential of molecularly targeted and MRD-directed therapies to achieve an individualized approach to patient management.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Altucci L, Rossin A, Raffelsberger W, Reitmair A, Chomienne C, Gronemeyer H. Retinoic acid-induced apoptosis in leukemia cells is mediated by paracrine action of tumor-selective death ligand TRAIL. Nat Med. 2001;7:680–686.
Altucci L, et al. Rexinoid-triggered differentiation and tumor-selective apoptosis of acute myeloid leukemia by protein kinase A-mediated desubordination of retinoid X receptor. Cancer Res. 2005;65:8754–8765.
Avvisati G, et al. AIDA (all-trans retinoic acid + idarubicin) in newly diagnosed acute promyelocytic leukemia: a Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (GIMEMA) pilot study. Blood. 1996;88:1390–1398.
Bennett JM, et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985;103:620–625.
Benoit G, et al. RAR-independent RXR signaling induces t(15;17) leukemia cell maturation. Embo J. 1999;18:7011–7018.
Burnett AK, Grimwade D, Solomon E, Wheatley K, Goldstone AH. Presenting white blood cell count and kinetics of molecular remission predict prognosis in acute promyelocytic leukemia treated with all-trans retinoic acid: result of the Randomized MRC Trial. Blood. 1999;93:4131–4143.
Carbone R, et al. Recruitment of the histone methyltransferase SUV39H1 and its role in the oncogenic properties of the leukemia-associated PML-retinoic acid receptor fusion protein. Mol Cell Biol. 2006;26:1288–1296.
Castaigne S, et al. All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I. Clinical results. Blood. 1990;76:1704–1709.
Catalano A, et al. The PRKAR1A gene is fused to RARA in a new variant acute promyelocytic leukemia. Blood. 2007;110:4073–4076.
Chambon P. A decade of molecular biology of retinoic acid receptors. Faseb J. 1996;10:940–954.
Chou WC, Jie C, Kenedy AA, Jones RJ, Trush MA, Dang CV. Role of NADPH oxidase in arsenic-induced reactive oxygen species formation and cytotoxicity in myeloid leukemia cells. Proc Natl Acad Sci USA. 2004;101:4578–4583.
Davison K, Mann KK, Waxman S, Miller WH, Jr. JNK activation is a mediator of arsenic trioxide-induced apoptosis in acute promyelocytic leukemia cells. Blood. 2004;103:3496–3502.
de Thé H, Chomienne C, Lanotte M, Degos L, Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature. 1990;347:558–561.
Degos L. The history of acute promyelocytic leukaemia. Br J Haematol. 2003;122:539–553.
Di Croce L, et al. Methyltransferase recruitment and DNA hypermethylation of target promoters by an oncogenic transcription factor. Science. 2002;295:1079–1082.
Douer D, Tallman MS. Arsenic trioxide: new clinical experience with an old medication in hematologic malignancies. J Clin Oncol. 2005;23:2396–2410.
Duprez E, Wagner K, Koch H, Tenen DG. C/EBPbeta: a major PML-RARA-responsive gene in retinoic acid-induced differentiation of APL cells. Embo J. 2003;22:5806–5816.
Estey E, et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood. 2006;107:3469–3473.
Falanga A, Rickles FR. Pathogenesis and management of the bleeding diathesis in acute promyelocytic leukaemia. Best Pract Res Clin Haematol. 2003;16:463–482.
Fenaux P, et al. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood. 1999;94:1192–1200.
Fenaux P, et al. Effect of all transretinoic acid in newly diagnosed acute promyelocytic leukemia. Results of a multicenter randomized trial. European APL 91 Group. Blood. 1993;82:3241–3249.
Frankel SR, Eardley A, Lauwers G, Weiss M, Warrell RP, Jr. The “retinoic acid syndrome” in acute promyelocytic leukemia. Ann Intern Med. 1992;117:292–296.
Gallagher RE. Retinoic acid resistance in acute promyelocytic leukemia. Leukemia. 2002;16:1940–1958.
Garcia-Manero G, et al. Phase I/II study of the combination of 5-aza-2′ -deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108:3271–3279.
Ghavamzadeh A, et al. Treatment of acute promyelocytic leukemia with arsenic trioxide without ATRA and/or chemotherapy. Ann Oncol. 2006;17:131–134.
Giafis N, et al. Role of the p38 mitogen-activated protein kinase pathway in the generation of arsenic trioxide-dependent cellular responses. Cancer Res. 2006;66:6763–6771.
Gore SD, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–6369.
Grignani F, et al. Fusion proteins of the retinoic acid receptor-alpha recruit histone deacetylase in promyelocytic leukaemia. Nature. 1998;391:815–818.
Grimwade D, et al. Characterization of acute promyelocytic leukemia cases lacking the classic t(15;17): results of the European Working Party. Groupe Francais de Cytogenetique Hematologique, Groupe de Francais d'Hematologie Cellulaire, UK Cancer Cytogenetics Group and BIOMED 1 European Community-Concerted Action “Molecular Cytogenetic Diagnosis in Haematological Malignancies”. Blood. 2000;96:1297–1308.
Grimwade D, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92:2322–2333.
Guidez F, Ivins S, Zhu J, Söderström M, Waxman S, Zelent A. Reduced retinoic acid-sensitivities of nuclear receptor corepressor binding to PML- and PLZF-RARalpha underlie molecular pathogenesis and treatment of acute promyelocytic leukemia. Blood. 1998;91:2634–2642.
Guillemin MC, et al. In vivo activation of cAMP signaling induces growth arrest and differentiation in acute promyelocytic leukemia. J Exp Med. 2002;196:1373–1380.
Hayakawa F, Privalsky ML. Phosphorylation of PML by mitogen-activated protein kinases plays a key role in arsenic trioxide-mediated apoptosis. Cancer Cell. 2004;5:389–401.
He LZ, et al. Distinct interactions of PML-RARalpha and PLZF-RARalpha with co-repressors determine differential responses to RA in APL. Nat Genet. 1998;18:126–135.
Head D, et al. Effect of aggressive daunomycin therapy on survival in acute promyelocytic leukemia. Blood. 1995;86:1717–1728.
Hong SH, Yang Z, Privalsky ML. Arsenic trioxide is a potent inhibitor of the interaction of SMRT corepressor with its transcription factor partners, including the PML-retinoic acid receptor alpha oncoprotein found in human acute promyelocytic leukemia. Mol Cell Biol. 2001;21:7172–7182.
Huang ME, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–572.
Joe Y, et al. ATR, PML, and CHK2 Play a Role in Arsenic Trioxide-induced Apoptosis. J Biol Chem. 2006;281:28764–28771.
Kamashev D, Vitoux D, de Thé H. PML-RARA-RXR oligomers mediate retinoid and rexinoid/cAMP cross-talk in acute promyelocytic leukemia cell differentiation. J Exp Med. 2004;199:1163–1174.
Kitamura K, Hoshi S, Koike M, Kiyoi H, Saito H, Naoe T. Histone deacetylase inhibitor but not arsenic trioxide differentiates acute promyelocytic leukaemia cells with t(11;17) in combination with all-trans retinoic acid. Br J Haematol. 2000;108:696–702.
Kondo T, Mori A, Darmanin S, Hashino S, Tanaka J, Asaka M. The seventh pathogenic fusion gene FIP1L1-RARA was isolated from a t(4;17)-positive acute promyelocytic leukemia. Haematologica. 2008;93:1414–1416.
Kurokawa R, et al. Polarity-specific activities of retinoic acid receptors determined by a co-repressor. Nature. 1995;377:451–454.
Kurokawa R, et al. Regulation of retinoid signalling by receptor polarity and allosteric control of ligand binding. Nature. 1994;371:528–531.
Kwok C, Zeisig BB, Dong S, So CW. Forced homo-oligomerization of RARalpha leads to transformation of primary hematopoietic cells. Cancer Cell. 2006;9:95–108.
Larson RS, Brown DC, Sklar LA. Retinoic acid induces aggregation of the acute promyelocytic leukemia cell line NB-4 by utilization of LFA-1 and ICAM-2. Blood. 1997;90:2747–2756.
Latagliata R, et al. Therapy-related myelodysplastic syndrome-acute myelogenous leukemia in patients treated for acute promyelocytic leukemia: an emerging problem. Blood. 2002;99:822–824.
Leoni F, et al. Arsenic trioxide therapy for relapsed acute promyelocytic leukemia: a bridge to transplantation. Haematologica. 2002;87:485–489.
Leung J, Pang A, Yuen WH, Kwong YL, Tse EW. Relationship of expression of aquaglyceroporin 9 with arsenic uptake and sensitivity in leukemia cells. Blood. 2007;109:740–746.
Lin RJ, Nagy L, Inoue S, Shao W, Miller WH, Evans RM. Role of the histone deacetylase complex in acute promyelocytic leukaemia. Nature. 1998;391:811–814.
Lin RJ, Evans RM. Acquisition of oncogenic potential by RAR chimeras in acute promyelocytic leukemia through formation of homodimers. Mol Cell. 2000;5:821–830.
Lobe I, et al. Myelodysplastic syndrome after acute promyelocytic leukemia: the European APL group experience. Leukemia. 2003;17:1600–1604.
Lu DP, et al. Tetra-arsenic tetra-sulfide for the treatment of acute promyelocytic leukemia: a pilot report. Blood. 2002;99:3136–3143.
Marasca R, et al. Missense mutations in the PML/RARalpha ligand binding domain in ATRA-resistant As(2)O(3) sensitive relapsed acute promyelocytic leukemia. Haematologica. 1999;84:963–968.
Marchetti M, Falanga A, Giovanelli S, Oldani E, Barbui T. All-trans-retinoic acid increases adhesion to endothelium of the human promyelocytic leukaemia cell line NB4. Br J Haematol. 1996;93:360–366.
Mathews V, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood. 2006;107:2627–2632.
McMullin MF, Nugent E, Thompson A, Hull D, Jones FG, Grimwade D. Prolonged molecular remission in PML-RARalpha-positive acute promyelocytic leukemia treated with minimal chemotherapy followed by maintenance including the histone deacetylase inhibitor sodium valproate. Leukemia. 2005;19:1676–1677.
Milligan DW, et al. Guidelines on the management of acute myeloid leukaemia in adults. Br J Haematol. 2006;135:450–474.
Minucci S, et al. Oligomerization of RAR and AML1 transcription factors as a novel mechanism of oncogenic activation. Mol Cell. 2000;5:811–820.
Mistry AR, Pedersen EW, Solomon E, Grimwade D. The molecular pathogenesis of acute promyelocytic leukaemia: implications for the clinical management of the disease. Blood Rev. 2003;17:71–97.
Paietta E, et al. Significantly lower P-glycoprotein expression in acute promyelocytic leukemia than in other types of acute myeloid leukemia: immunological, molecular and functional analyses. Leukemia. 1994;8:968–973.
Rosenfeld MG, Glass CK. Coregulator codes of transcriptional regulation by nuclear receptors. J Biol Chem. 2001;276:36865–36868.
Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–1428.
Sanz MA, et al. A modified AIDA protocol with anthracycline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RARalpha-positive acute promyelocytic leukemia. PETHEMA group. Blood. 1999;94:3015–3021.
Sanz MA, Fenaux P, Lo Coco F. Arsenic trioxide in the treatment of acute promyelocytic leukemia. A review of current evidence. Haematologica. 2005;90:1231–1235.
Sanz MA, Tallman MS, Lo-Coco F. Tricks of the trade for the appropriate management of newly diagnosed acute promyelocytic leukemia. Blood. 2005;105:3019–3025.
Shen ZX, et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2004;101:5328–5335.
Soignet SL, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341–1348.
Soignet SL, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–3860.
Sternsdorf T, et al. Forced retinoic acid receptor alpha homodimers prime mice for APL-like leukemia. Cancer Cell. 2006;9:81–94.
Tallman MS, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337:1021–1028.
Verma A, et al. Activation of Rac1 and the p38 mitogen-activated protein kinase pathway in response to arsenic trioxide. J Biol Chem. 2002;277:44988–44995.
Villa R, et al. The methyl-CpG binding protein MBD1 is required for PML-RARalpha function. Proc Natl Acad Sci USA. 2006;103:1400–1405.
Warrell RP, Jr, He LZ, Richon V, Calleja E, Pandolfi PP. Therapeutic targeting of transcription in acute promyelocytic leukemia by use of an inhibitor of histone deacetylase. J Natl Cancer Inst. 1998;90:1621–1625.
Warrell RP. Retinoid resistance in acute promyelocytic leukemia:new mechanisms, strategies and implications. Blood. 1993;82:2175–2181.
Zheng PZ, et al. Systems analysis of transcriptome and proteome in retinoic acid/arsenic trioxide-induced cell differentiation/apoptosis of promyelocytic leukemia. Proc Natl Acad Sci U S A. 2005;102:7653–7658.
Zhou DC, Kim SH, Ding W, Schultz C, Warrell RP, Gallagher RE. Frequent mutations in the ligand-binding domain of PML-RARalpha after multiple relapses of acute promyelocytic leukemia: analysis for functional relationship to response to all-trans retinoic acid and histone deacetylase inhibitors in vitro and in vivo. Blood. 2002;99:1356–1363.
Zhou J, Pérès L, Honoré N, Nasr R, Zhu J, de Thé H. Dimerization-induced corepressor binding and relaxed DNA-binding specificity are critical for PML/RARA induced immortalization. Proc Natl Acad Sci USA. 2006;103:9238–9243.
Acknowledgments
DG and FG gratefully acknowledge grant support from the Leukaemia Research Fund of Great Britain. DG is also supported by the European LeukemiaNet.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Grimwade, D., Mistry, A.R., Solomon, E., Guidez, F. (2009). Acute Promyelocytic Leukemia: A Paradigm for Differentiation Therapy. In: Nagarajan, L. (eds) Acute Myelogenous Leukemia. Cancer Treatment and Research, vol 145. Springer, New York, NY. https://doi.org/10.1007/978-0-387-69259-3_13
Download citation
DOI: https://doi.org/10.1007/978-0-387-69259-3_13
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-0-387-69257-9
Online ISBN: 978-0-387-69259-3
eBook Packages: MedicineMedicine (R0)