Abstract
Background
The pharmacokinetic properties of the immediate-release (IR) and the recently developed controlled-release (CR) formulation of pregabalin are dose proportional. Pregabalin IR can be taken with or without food.
Objectives
This analysis characterizes the effect of food on pregabalin CR. The objectives of this analysis were: (1) to evaluate the effect of administration time and fat or caloric content of an accompanying meal on the pharmacokinetic properties of a single dose of pregabalin CR (330 mg) relative to a single dose of pregabalin IR (300 mg); (2) to evaluate the pharmacokinetic properties of a single dose of pregabalin CR administered fasted relative to a single dose of pregabalin CR administered immediately after food; and (3) to determine the safety and tolerability of single-dose administration of pregabalin CR and IR with and without food.
Methods
The effect of food on the pharmacokinetic properties of pregabalin CR was determined in five phase I, open-label, single-dose, crossover studies (24–28 participants/study). Caloric and fat content of meals were varied and treatments were administered in the morning, at midday, or in the evening. Blood samples were collected up to 48 h post-dose. Pharmacokinetic parameters were estimated from plasma concentration–time data using standard noncompartmental methods. Adverse events were monitored throughout all studies.
Results
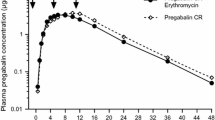
One hundred and twenty-eight healthy participants (19–54 years of age) received pregabalin. Peak plasma concentrations (C max) were lower for CR than the respective pregabalin IR doses, and time to C max occurred later. When pregabalin CR was administered with food at midday or in the evening, total exposures [area under the plasma concentration–time curve from time zero extrapolated to infinite time (AUC∞)] were equivalent for pregabalin CR and IR formulations regardless of fat or caloric content. When pregabalin CR was administered with an 800–1,000 calorie medium-fat breakfast, AUC∞ was equivalent for pregabalin CR and IR. Bioequivalence criteria for comparison of pregabalin CR after a low- or medium-calorie breakfast relative to pregabalin IR were not met; however, bioavailability of the pregabalin CR vs. IR formulation was relatively high (75–86 %). When pregabalin CR was administered fasted, the AUC∞ was 70–78 % of the AUC∞ of pregabalin CR administered with food and bioequivalence criteria were not met. Additionally, the AUC∞ of the pregabalin CR formulation administered fasted was 62–69 % of that of pregabalin IR administered fasted and bioequivalence criteria were not met. Single-dose pregabalin CR and IR were well tolerated in all studies, with no serious or severe adverse events reported.
Conclusion
Time of day of administration and the fat and caloric content of the accompanying meal had minimal overall effect on the pharmacokinetic properties and bioavailability of the pregabalin CR formulation.



Similar content being viewed by others
References
Lyrica (pregabalin) [package insert]. Pfizer Inc, New York. 2013. http://labeling.pfizer.com/ShowLabeling.aspx?id=561. Accessed 11 Jun 2014.
Lyrica EU product information. Pfizer Limited, Sandwich. 2009. http://www.emea.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000546/WC500046602.pdf. Accessed 11 Jun 2014.
Bockbrader HN, Radulovic LL, Posvar EL, et al. Clinical pharmacokinetics of pregabalin in healthy volunteers. J Clin Pharmacol. 2010;50:941–50.
Corrigan BW, Pool WF, Posvar EL, et al. Metabolic disposition of pregabalin in healthy volunteers [abstract PI-68]. Clin Pharmacol Ther. 2001;69:P18.
Srivastava K, Arora A, Kataria A, et al. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysis. Patient Prefer Adherence. 2013;7:419–34.
Bockbrader HN, Burger P, Knapp L, Corrigan BW. Population pharmacokinetics of pregabalin in healthy subjects and patients with chronic pain or partial seizures. Epilepsia. 2011;52:248–57.
Alebic-Kolbah T. Pregabalin: development and validation of an LC-MS/MS method for pediatric studies [abstract TP542]. J Am Soc Mass Spectrom. 2012;23:109.
Kellow JE, Borody TJ, Phillips SF, et al. Human interdigestive motility: variations in patterns from esophagus to colon. Gastroenterology. 1986;91:386–95.
Kumar D, Wingate D, Ruckebusch Y. Circadian variation in the propagation velocity of the migrating motor complex. Gastroenterology. 1986;91:926–30.
Wilson P, Perdikis G, Hinder RA, et al. Prolonged ambulatory antroduodenal manometry in humans. Am J Gastroenterol. 1994;89:1489–95.
Soffer EE, Thongsawat S, Ellerbroek S. Prolonged ambulatory duodeno-jejunal manometry in humans: normal values and gender effect. Am J Gastroenterol. 1998;93:1318–23.
Freynhagen R, Serpell M, Emir B, et al. A comprehensive drug safety evaluation of pregabalin in peripheral neuropathic pain. Pain Pract. 2013. doi:10.1111/papr.12146.
Chew ML, Alvey CW, Plotka A, et al. Pregabalin controlled-release pharmacokinetics in healthy volunteers: analysis of four multiple-dose randomized clinical pharmacology studies. Clin Drug Investig. 2014. doi:10.1007/s40261-014-0221-2.
Brodie MJ, Wilson EA, Wesche DL, et al. Pregabalin drug interaction studies: lack of effect on the pharmacokinetics of carbamazepine, phenytoin, lamotrigine, and valproate in patients with partial epilepsy. Epilepsia. 2005;46:1407–13.
Bockbrader HN, Burger P, Knapp L. Pregabalin effect on steady-state pharmacokinetics of carbamazepine, lamotrigine, phenobarbital, phenytoin, topiramate, valproate, and tiagabine. Epilepsia. 2011;52:405–9.
Acknowledgments
The studies described in this paper were sponsored by Pfizer Inc, who was involved in the study design, the collection, analysis, and interpretation of the data, the writing of the report, and the decision to submit the paper for publication. Medical writing support was provided by Lorna Forse, PhD, of Engage Scientific Solutions and funded by Pfizer Inc.
Conflict of interest
Marci L. Chew, Anna Plotka, Christine W. Alvey, Verne W. Pitman, Tanja Alebic-Kolbah, and Joseph M. Scavone are all full-time employees of Pfizer Inc and hold stock in Pfizer Inc. Howard N. Bockbrader was an employee of Pfizer Inc at the time these studies were conducted and holds stock in Pfizer Inc.
Ethical statement
All studies were conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with the International Conference on Harmonisation Good Clinical Practice guidelines. All participants provided written informed consent before entering a study. The final protocols and informed consent documentation were reviewed and approved by the ethics committees of either Integreview (Austin, TX, USA) or the Midlands Institutional Review Board (Overland Park, KS, USA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chew, M.L., Plotka, A., Alvey, C.W. et al. Pharmacokinetics of Pregabalin Controlled-Release in Healthy Volunteers: Effect of Food in Five Single-Dose, Randomized, Clinical Pharmacology Studies. Clin Drug Investig 34, 617–626 (2014). https://doi.org/10.1007/s40261-014-0211-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-014-0211-4