Abstract
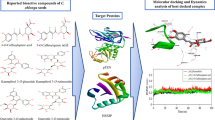
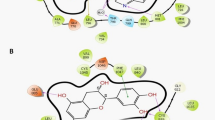
The hepatitis B virus × protein (HBx) of Hepatitis B virus activates AP-1 protein and causes the down regulation of PTEN (full name) and p53 leads to the tumor formation in liver. In this study, the interactions between DNA and AP-1 have been targeted by docking of natural compounds epigallocatechine gallate (EGCG), curcumin, luteoline, genistein, ellagic acid, resveratrol, lupeol, betulinic acid and lycopene. Green Tea (Camellia sinensis) possesses anticancer property. EGCG obtained from green tea shows favourable binding with AP-1 protein among all natural compounds. It is necessary to target DNA binding domain which binds at DNA to induce the expression of p53 and PTEN gene. EGCG have shown interaction at these positions, which may minimize down regulation of p53 and PTEN gene. To increase the binding affinity and bioavailability of EGCG, derivatives have been designed by mimicking the position of H and OH group. One of acetylated derivative EGCG15, made by replacing OH group by OCOCH3 shows better affinity than other derivatives and binds at amino acid Asp 163(G), Ser 278(F), Lys 282(F), Arg 288(F) with six hydrogen bonds and −5.60 kcal/mol energy. Affinity of EGCG with AP-1 protein was greatly enhanced in methoxy derivative EGCG05 which binds at Asp 163, Asp 170, Ser 278, Arg 281, and Arg 288 with eight hydrogen bonds and −6.30 kcal/mol energy (energy complex). Substitution of OH by OCOCH3 increases bioavailability after sequential addition at various positions in EGCG leads in silico. These derivatives also show better affinity, but less than methoxy (OCH3) derivative. Substitution by OCH3 leads to slight increment of 6 % in oral absorption. Substitution by NH2 group leads to no changes in oral absorption or bioavailability. This may lead to inhibition of AP-1 protein which down regulates tumor suppressor gene p53 and PTEN.





Similar content being viewed by others
References
Amin AR, Kucuk O, Khuri FR, Shin DM (2009) Perspectives for cancer prevention with natural compounds. J Clin Oncol 27(16):2712–2725
Auger C, Mullen W, Hara Y, Crozier A (2008) Bioavailability of polyphenon E flavan-3-ols in humans with an ileostomy. J Nutr 138(8):1535S–1542S
Barthelman M, Bair WB 3rd, Stickland KK, Chen W, Timmermann BN, Valcic S, Dong Z, Bowden GT (1998) (−)-Epigallocatechin-3-gallate inhibition of ultraviolet B-induced AP-1 activity. Carcinogenesis 19(12):2201–2204
Benn J, Su F, Doria M, Schneider RJ (1996) Hepatitis B virus HBx protein induces transcription factor AP-1 by activation of extracellular signal-regulated and c-Jun N-terminal mitogen-activated protein kinases. J Virol 70(8):4978–4985
Bierhaus A, Zhang Y, Quehenberger P, Luther T, Haase M, Müller M, Mackman N, Ziegler R, Nawroth PP (1997) The dietary pigment curcumin reduces endothelial tissue factor gene expression by inhibiting binding of AP-1 to the DNA and activation of NF-kappa B. Thromb Haemost 77(4):772–782
Bode AM, Dong Z (2004) Targeting signal transduction pathways by chemopreventive agents. Mutat Res 555(1–2):33–51
Bouchard MJ, Wang L, Schneider RJ (2006) Activation of focal adhesion kinase by hepatitis B virus HBx protein: multiple functions in viral replication. J Virol 80(9):4406–4414
Chung TW, Lee YC, Ko JH, Kim CH (2003) Hepatitis B Virus X protein modulates the expression of PTEN by inhibiting the function of p53, a transcriptional activator in liver cells. Cancer Res 63(13):3453–3458
Collins QF, Liu HY, Pi J, Liu Z, Quon MJ, Cao W (2007) Epigallocatechin-3 gallate(EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J Biol Chem 282(41):30143–30149
Colmenarejo G, Alvarez-Pedraglio A, Lavandera JL (2001) Cheminformatic models to predict binding affinities to human serum albumin. J Med Chem 44(25):4370–4378
Dong Z, Ma W, Huang C, Yang CS (1997) Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols, (−)-epigallocatechin gallate, and theaflavins. Cancer Res 57(19):4414–4419
Dube A, Nicolazzo JA, Larson I (2010) Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallate. Eur J Pharm Sci 41(2):219–225
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47(7):1739–1749
Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT (2006) Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem 49(21):6177–6196
Fujiki H (1999) Two stages of cancer prevention with green tea. J Cancer Res Clin Oncol 125(11):589–597
Fujiki H, Suganuma M, Okabe S, Sueoka E, Suga K, Imai K, Nakachi K (2000) A new concept of tumor promotion by tumor necrosis factor-alpha, and cancer preventive agents (−)-epigallocatechin gallate and green tea–a review. Cancer Detect Prev 24(1):91–99
Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL (2004) Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem 47(7):1750–1759
Haviv I, Shamay M, Doitsh G, Shaul Y (1998) Hepatitis B virus pX targets TFIIB in transcription coactivation. Mol Cell Biol 18(3):1562–1569
Hong Byun E, Fujimura Y, Yamada K, Tachibana H (2010) TLR4 signaling inhibitory pathway induced by green tea polyphenol epigallocatechin-3-gallate through 67-kDa laminin receptor. J Immunol 185(1):33–45
Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH (1991) Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res 51(3):813–819
Huang C, Ma WY, Li J, Dong Z (1999) Arsenic induces apoptosis through a c-Jun NH2-terminal kinase-dependent, p53-independent pathway. Cancer Res 59(13):3053–3058
Imai K, Suga K, Nakachi K (1997) Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med 26(6):769–775
Jorgensen WL, Duffy EM (2000) Prediction of drug solubility from Monte Carlo simulations. Bioorg Med Chem Lett 10(11):1155–1158
Jorgensen WL, Duffy EM (2002) Prediction of drug solubility from structure. Adv Drug Deliv Rev 54(3):355–366
Kanwar J, Taskeen M, Mohammad I, Huo C, Chan TH, Dou QP (2012) Recent advances on tea polyphenols. Front Biosci (Elite Ed) 4:111–131
Karin M, Liu ZG, Zandi E (1997) AP-1 function and regulation. Curr Opin Cell Biol 9:240–246
Katiyar SK, Mohan RR, Agarwal R, Mukhtar H (1997) Protection against induction of mouse skin papillomas with low and high risk of conversion to malignancy by green tea polyphenols. Carcinogenesis 18(3):497–502
Kida K, Suzuki M, Matsumoto N, Nanjo F, Hara Y (2000) Identification of biliary metabolites of (−)-epigallocatechin gallate in rats. J Agric Food Chem 48(9):4151–4155
Kim HS, Kim MH, Jeong M, Hwang YS, Lim SH, Shin BA, Ahn BW, Jung YD (2004) EGCG blocks tumor promoter-induced MMP-9 expression via suppression of MAPK and AP-1 activation in human gastric AGS cells. Anticancer Res 24(2B):747–753
Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47(7):1739–1749
Lambert JD, Hong J, Kim DH, Mishin VM, Yang CS (2004) Piperine enhances the bioavailability of the tea polyphenol (−)-epigallocatechin-3-gallate in mice. J Nutr 134(8):1948–1952
Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS (2002) Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomark Prev 11:1025–1032
Li J, Zhang D, Stoner GD, Huang C (2008) Differential effects of black raspberry and strawberry extracts on BaPDE-induced activation of transcription factors and their target genes. Mol Carcinog 47(4):286–294
Li N, Taylor LS, Mauer LJ (2011) Degradation kinetics of catechins in green tea powder: effects of temperature and relative humidity. J Agric Food Chem 59(11):6082–6090
Lorenz M, Jochmann N, von Krosigk A, Martus P, Baumann G, Stangl K, Stangl V (2007) Addition of milk prevents vascular protective effects of tea. Eur Heart J 28(2):219–223
Lu H, Meng X, Yang CS (2003) Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate. Drug Metab Dispos 31(5):572–579
Matsumoto Y, Kaihatsu K, Nishino K, Ogawa M, Kato N, Yamaguchi A (2012) Antibacterial and antifungal activities of new acylated derivatives of epigallocatechin gallate. Front Microbiol 16(3):53
Mereles D, Hunstein W (2011) Epigallocatechin-3-gallate (EGCG) for clinical trials: more pitfalls than promises? Int J Mol Sci 12(9):5592–5603
Mukhtar H, Ahmad N (1999) Mechanism of cancer chemopreventive activity of green tea. Proc Soc Exp Biol Med 220:234–238
Nomura M, Ma W-Y, Chen N, Bode AM, Dong Z (2000) Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced NF-κB activation by tea polyphenols, (−)-epigallocatechin gallate and theaflavins. Carcinogenesis 21(10):1885–1890
Prusty BK, Das BC (2005) Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin. Int J Cancer 113(6):951–960
Roowi S, Stalmach A, Mullen W, Lean ME, Edwards CA, Crozier A (2010) Green tea flavan-3-ols: colonic degradation and urinary excretion of catabolites by humans. J Agric Food Chem 58(2):1296–1304
Singh R, Ahmed S, Islam N, Goldberg VM, Haqqi TM (2002) Epigallocatechin-3-gallate inhibits interleukin-1 beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: suppression of nuclear factor kappaB activation by degradation of the inhibitor of nuclear factor kappaB. Arthritis Rheum 46(8):2079–2086
Singh R, Ahmed S, Malemud CJ, Goldberg VM, Haqqi TM (2003) Epigallocatechin-3-gallate selectively inhibits interleukin-1 beta-induced activation of mitogen activated protein kinase subgroup c-Jun N-terminal kinase in human osteoarthritis chondrocytes. J Orthop Res 21(1):102–109
Stalmach A, Troufflard S, Serafini M, Crozier A (2009) Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol Nutr Food Res 53(Suppl 1):S44–S53
Surh Y (1999) Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res 428(1–2):305–327
Tanaka H, Miyoshi H, Chuang YC, Ando Y, Takahashi T (2007) Solid-phase synthesis of epigallocatechin gallate derivatives. Angew Chem Int Ed Engl 46(31):5934–5937
Vyas S, Sharma M, Sharma PD, Singh TV (2007) Design, semisynthesis, and evaluation of O-acyl derivatives of (−)-epigallocatechin-3-gallate as antitumor agents. J Agric Food Chem 55(15):6319–6324
Wang XW, Forrester K, Yeh H, Feitelson MA, Gu JR, Harris CC (1994) Hepatitis B virus X protein inhibits p53 sequence-specific DNA binding, transcriptional activity, and association with transcription factor ERCC3. Proc Natl Acad Sci USA 91(6):2230–2234
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270(5240):1326–1331
Yang CS, Chung JY, Yang G, Chhabra SK, Lee MJ (2000) Tea and tea polyphenols in cancer prevention. J Nutr 130(2):4725–4785
Yang F, Oz HS, Barve S, de Villiers WJ, McClain CJ, Varilek GW (2001) The green tea polyphenol (−)-epigallocatechin-3-gallate blocks nuclear factor-kappa B activation by inhibiting I kappa B kinase activity in the intestinal epithelial cell line IEC-6. Mol Pharmacol 60(3):528–533
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sagar, M., Pathak, R.K., Pandey, R.K. et al. Binding affinity analysis and ADMET prediction of epigallocatechine gallate (EGCG) derivatives for AP-1 protein: a drug target for liver cancer. Netw Model Anal Health Inform Bioinforma 3, 66 (2014). https://doi.org/10.1007/s13721-014-0066-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13721-014-0066-x