Abstract
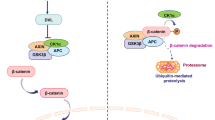
The Wnt signaling pathway regulates some of the crucial aspects of cellular processes. The beta-catenin dependent Wnt signaling (Wnt/β-catenin) pathway controls the expression of key developmental genes, and acts as an intracellular signal transducer. The association of Wnt/β-catenin pathway is often reported with different cancers. In this study, we have reviewed the association of Wnt/β-catenin pathway with bone cancers, focusing on carcinogenesis and therapeutic aspects. Wnt/β-catenin pathway is a highly complex and unique signaling pathway, which has ability to regulate gene expression, cell invasion, migration, proliferation, and differentiation for the initiation and progression of bone cancers, especially osteosarcoma. Association of Wnt/β-catenin pathway with chondrosarcoma, Ewing’s sarcoma and chondroma is also documented. Recently, targeting Wnt/β-catenin pathway has gained significant interests as a potential therapeutic application for the treatment of bone cancers. Small RNA technology to knockdown aberrant Wnt/β-catenin or inhibition of β-catenin expression by natural component has shown promising effects against bone cancers. Advances in understanding the mechanisms of Wnt signaling and new technologies have facilitated the discovery of agents that can target and regulate Wnt/β-catenin signaling pathway, and these may provide a basement for the innovative therapeutic approaches in the treatment of bone cancers.


Similar content being viewed by others
References
MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26.
Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75.
Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–98.
Rao TP, Kühl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798–806.
Mao J, Wang J, Liu B, Pan W, Farr 3rd GH, Flynn C, et al. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–9.
Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, et al. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135:367–75.
He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–77.
Sakanaka C, Sun TQ, Williams LT. New steps in the Wnt/beta-catenin signal transduction pathway. Recent Prog Horm Res. 2000;55:225–36.
Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26.
Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–22.
Marques C, Ferreira JM, Andronescu E, Ficai D, Sonmez M, Ficai A. Multifunctional materials for bone cancer treatment. Int J Nanomedicine. 2014;9:2713–25.
Rosenberg AE. Robins and cotran pathologic basis of disease. Bone, joints and soft tissue tumors. 8th ed. Philadelphia: WB Saunders; 2010. p. 1203–56.
Kundu ZS. Classification, imaging, biopsy and staging of osteosarcoma. Indian J Orthop. 2014;48:238–46.
Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13.
Calvert GT, Randall RL, Jones KB, Cannon-Albright L, Lessnick S, Schiffman JD. At-risk populations for osteosarcoma: the syndromes and beyond. Sarcoma. 2012;2012:152382.
Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, et al. Children’s Oncology Group. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival—a report from the Children’s Oncology Group. J Clin Oncol. 2008;26:633–8.
Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–90.
Sakamoto A. The molecular pathogenesis of dedifferentiated chondrosarcoma. Indian J Orthop. 2014;48:262–5.
Darouassi Y, Touati MM, Chihani M, Nadour K, Boussouga M, Ammar H, et al. Chondrosarcoma metastasis in the thyroid gland: a case report. J Med Case Rep. 2014;8:157.
Sridhar H, Vijaya M, Clement W, Srinivas C. Chondrosarcoma arising in an enchondroma of the metacarpal bone—a case report. J Clin Diagn Res. 2014;8:142–3.
Lin PP, Moussallem CD, Deavers MT. Secondary chondrosarcoma. J Am Acad Orthop Surg. 2010;18:608–15.
Geng S, Zhang J, Zhang LW, Wu Z, Jia G, Xiao X, et al. Diagnosis and microsurgical treatment of chondromas and chondrosarcomas of the cranial base. Oncol Lett. 2014;8:301–4.
Fiorenza F, Abudu A, Grimer RJ, Carter SR, Tillman RM, Ayoub K, et al. Risk factors for survival and local control in chondrosarcoma of bone. J Bone Joint Surg (Br). 2002;84:93–9.
Chen B, Yang Y, Chen L, Zhou F, Yang H. Unilateral lateral mass fixation of cervical spinal low-grade chondrosarcoma with intralesional resection: a case report. Oncol Lett. 2014;7:1515–8.
Rossig C. Cellular immunotherapy strategies for Ewing sarcoma. Immunotherapy. 2014;6:611–21.
Iwamoto Y. Diagnosis and treatment of Ewing’s sarcoma. Jpn J Clin Oncol. 2007;37:79–89.
Cheung MR. Optimization of predictors of Ewing sarcoma cause-specific survival: a population study. Asian Pac J Cancer Prev. 2014;15:4143–5.
Bernstein M, Kovar H, Paulussen M, Randall RL, Schuck A, Teot LA, et al. Ewing’s sarcoma family of tumors: current management. Oncologist. 2006;11:503–19.
Owen LA, Kowalewski AA, Lessnick SL. EWS/FLI mediate transcriptional repression via NKX2.2 during oncogenic transformation in Ewing’s sarcoma. PLoS One. 2008;3:e1965.
Choi Y, Choi H, Jin KS, Oh JH. A case of auricular chondroma. Korean J Audiol. 2013;17:156–8.
Choi Y, Lim WS, Lee AY, Lee SH. Extraskeletal chondroma of the scalp: an atypical location. Indian J Dermatol Venereol Leprol. 2013;79:435–6.
Chung EB, Enzinger FM. Chondroma of soft parts. Cancer. 1978;41:1414–24.
Gungor S, Kamali G, Canat D, Gokdemir G. Soft tissue chondroma of the index finger: clinical, histological and radiological findings in a unique case. Dermatol Online J. 2013;19:18176.
Rabbani SA, Arakelian A, Farookhi R. LRP5 knockdown: effect on prostate cancer invasion growth and skeletal metastasis in vitro and in vivo. Cancer Med. 2013;2:625–35.
Chu T, Teng J, Jiang L, Zhong H, Han B. Lung cancer-derived Dickkopf1 is associated with bone metastasis and the mechanism involves the inhibition of osteoblast differentiation. Biochem Biophys Res Commun. 2014;443:962–8.
Iwaya K, Ogawa H, Kuroda M, Izumi M, Ishida T, Mukai K. Cytoplasmic and/or nuclear staining of beta-catenin is associated with lung metastasis. Clin Exp Metastasis. 2003;20:525–9.
Lin CH, Guo Y, Ghaffar S, McQueen P, Pourmorady J, Christ A, et al. Dkk-3, a secreted wnt antagonist, suppresses tumorigenic potential and pulmonary metastasis in osteosarcoma. Sarcoma. 2013;2013:147541.
Cai Y, Cai T, Chen Y. Wnt pathway in osteosarcoma, from oncogenic to therapeutic. J Cell Biochem. 2014;115:625–31.
Lin CH, Ji T, Chen CF, Hoang BH. Wnt signaling in osteosarcoma. Adv Exp Med Biol. 2014;804:33–45.
Ma Y, Ren Y, Han EQ, Li H, Chen D, Jacobs JJ, et al. Inhibition of the Wnt-β-catenin and Notch signaling pathways sensitizes osteosarcoma cells to chemotherapy. Biochem Biophys Res Commun. 2013;431:274–9.
Kansara M, Tsang M, Kodjabachian L, Sims NA, Trivett MK, Ehrich M, et al. Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J Clin Invest. 2009;119:837–51.
Cai Y, Mohseny AB, Karperien M, Hogendoorn PC, Zhou G, Cleton-Jansen AM. Inactive Wnt/beta-catenin pathway in conventional high-grade osteosarcoma. J Pathol. 2010;220:24–33.
Wan Y, Zhao W, Jiang Y, Liu D, Meng G, Cai Y. β-catenin is a valuable marker for differential diagnosis of osteoblastoma and osteosarcoma. Hum Pathol. 2014;45:1459–65.
Du X, Yang J, Yang D, Tian W, Zhu Z. The genetic basis for inactivation of Wnt pathway in human osteosarcoma. BMC Cancer. 2014;14:450.
Fanburg-Smith JC, Auerbach A, Marwaha JS, Wang Z, Rushing EJ. Reappraisal of mesenchymal chondrosarcoma: novel morphologic observations of the hyaline cartilage and endochondral ossification and beta-catenin, Sox9, and osteocalcin immunostaining of 22 cases. Hum Pathol. 2010;41:653–62.
Uren A, Wolf V, Sun YF, Azari A, Rubin JS, Toretsky JA. Wnt/Frizzled signaling in Ewing sarcoma. Pediatr Blood Cancer. 2004;43:243–9.
Endo Y, Beauchamp E, Woods D, Taylor WG, Toretsky JA, Uren A, et al. Wnt-3a and Dickkopf-1 stimulate neurite outgrowth in Ewing tumor cells via a Frizzled3- and c-Jun N-terminal kinase-dependent mechanism. Mol Cell Biol. 2008;28:2368–79.
Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–38.
Yuasa T, Kondo N, Yasuhara R, Shimono K, Mackem S, Pacifici M, et al. Transient activation of Wnt/{beta}-catenin signaling induces abnormal growth plate closure and articular cartilage thickening in postnatal mice. Am J Pathol. 2009;175:1993–2003.
Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–50.
Cantley L, Saunders C, Guttenberg M, Candela ME, Ohta Y, Yasuhara R, et al. Loss of β-catenin induces multifocal periosteal chondroma-like masses in mice. Am J Pathol. 2013;182:917–27.
Richardson RB. Age-specific bone tumour incidence rates are governed by stem cell exhaustion influencing the supply and demand of progenitor cells. Mech Ageing Dev. 2014;139C:31–40.
Jin S, Shen JN, Wang J, Huang G, Zhou JG. Oridonin induced apoptosis through Akt and MAPKs signaling pathways in human osteosarcoma cells. Cancer Biol Ther. 2007;6:261–8.
Liu Y, Liu YZ, Zhang RX, Wang X, Meng ZJ, Huang J, et al. Oridonin inhibits the proliferation of human osteosarcoma cells by suppressing Wnt/β-catenin signaling. Int J Oncol. 2014;45:795–803.
Zhang F, Chen A, Chen J, Yu T, Guo F. SiRNA-mediated silencing of beta-catenin suppresses invasion and chemosensitivity to doxorubicin in MG-63 osteosarcoma cells. Asian Pac J Cancer Prev. 2011;12:239–45.
Xia JJ, Pei LB, Zhuang JP, Ji Y, Xu GP, Zhang ZP, et al. Celecoxib inhibits β-catenin-dependent survival of the human osteosarcoma MG-63 cell line. J Int Med Res. 2010;38:1294–304.
Leow PC, Tian Q, Ong ZY, Yang Z, Ee PL. Antitumor activity of natural compounds, curcumin and p KF118–310, as Wnt/β-catenin antagonists against human osteosarcoma cells. Investig New Drugs. 2010;28:766–82.
Leow PC, Bahety P, Boon CP, Lee CY, Tan KL, Yang T, et al. Functionalized curcumin analogs as potent modulators of the Wnt/β-catenin signaling pathway. Eur J Med Chem. 2014;71:67–80.
Liu Y, Wang W, Xu J, Li L, Dong Q, Shi Q, et al. Dihydroartemisinin inhibits tumor growth of human osteosarcoma cells by suppressing Wnt/β-catenin signaling. Oncol Rep. 2013;30:1723–30.
Zeng L, Wang W, Rong XF, Zhong Y, Jia P, Zhou GQ, et al. Chondroprotective effects and multi-target mechanisms of Icariin in IL-1 beta-induced human SW 1353 chondrosarcoma cells and a rat osteoarthritis model. Int Immunopharmacol. 2014;18:175–81.
Fuerer C, Habib SJ, Nusse R. A study on the interactions between heparin sulfate proteoglycans and Wnt proteins. Dev Dyn. 2010;239:184–90.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81201420, 81272034), the Provincial Science Foundation of Hunan (No. 14JJ3032), the Scientific Research Project of the Development and Reform Commission of Hunan Province ([2013]1199), the Scientific Research Project of Science and Technology Office of Hunan Province (2013SK2018), and the Doctoral Scientific Fund Project of the Ministry of Education of China (20120162110036).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tian, J., He, H. & Lei, G. Wnt/β-catenin pathway in bone cancers. Tumor Biol. 35, 9439–9445 (2014). https://doi.org/10.1007/s13277-014-2433-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2433-8