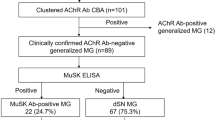
Abstract
Objective
To evaluate the frequency, distribution and clinical significance of the antibodies to the fetal and/or adult acetylcholine receptor (AChR) in patients with myasthenia gravis (MG).
Methods
AChR antibodies were detected by cell-based assay in the serum of ocular MG (OMG) (n = 90) and generalized MG (GMG) patients (n = 110). The fetaltype (2α: β: Γ: δ) and adult-type (2α: β: ɛ: δ) AChR were used as antigens, and their relevance to disease presentation was assessed.
Results
The overall frequencies of anti-adult and anti-fetal AChR antibodies were similar in all 200 patients examined, with 14 having serum specific to the AChR-Γ subunit, and 22 to the AChR-ɛ subunit. The overall sensitivity when using the fetal and adult AChR antibodies was higher than that when using the fetal AChR antibody only (P = 0.015). Compared with OMG patients, the mean age at disease onset and the positive ratio of antibodies to both isoforms of the AChR were significantly higher in patients who subsequently progressed to GMG. Older patients and patients with both anti-fetal and anti-adult AChR antibodies had a greater risk for developing generalized disease [odds ratio (OR), 1.03; 95% confidence interval (CI), 1.01–1.06 and OR, 5.09; 95% CI, 2.23–11.62].
Conclusion
Using both fetal- and adult-type AChRs as the antigens may be more sensitive than using either subtype. Patients with serum specific to both isoforms are at a greater risk of progressing to GMG. Patients with disease onset at an advanced age appear to have a higher frequency of GMG conversion.
Similar content being viewed by others
References
Vincent A, Palace J, Hilton-Jones D. Myasthenia gravis. Lancet 2001, 357: 2122–2128.
Yang L, Maxwell S, Leite MI, Waters P, Clover L, Fan X, et al. Non-radioactive serological diagnosis of myasthenia gravis and clinical features of patients from Tianjin, China. J Neurol Sci 2011, 301: 71–76.
Barton JJ, Fouladvand M. Ocular aspects of myasthenia gravis. Semin Neurol 2000, 20: 7–20.
Kupersmith MJ. Ocular myasthenia gravis: treatment successes and failures in patients with long-term follow-up. J Neurol 2009, 256: 1314–1320.
Kusner LL, Puwanant A, Kaminski HJ. Ocular myasthenia: diagnosis, treatment, and pathogenesis. Neurologist 2006, 12: 231–239.
Grob D. Natural history of myasthenia gravis. In: Engel AG (Ed.). Myasthenia Gravis and Myasthenic Disorders. New York, NY: Oxford University Press, 1999: 135–136.
Luchanok U, Kaminski HJ. Ocular myasthenia: diagnostic and treatment recommendations and the evidence base. Curr Opin Neurol 2008, 21: 8–15.
Na SJ, So SH, Lee KO, Choi YC. Elevated serum level of interleukin-32α in the patients with myasthenia gravis. J Neurol 2011, 258: 1865–1870.
Hong YH, Kwon SB, Kim BJ, Kim BJ, Kim SH, Kim JK, et al. Prognosis of ocular myasthenia in Korea: a retrospective multicenter analysis of 202 patients. J Neurol Sci 2008, 273: 10–14.
MacLennan C, Beeson D, Buijs AM, Vincent A, Newsom-Davis J. Acetylcholine receptor expression in human extraocular muscles and their susceptibility to myasthenia gravis. Ann Neurol 1997, 41: 423–431.
Ohta K, Fujinami A, Saida T, Nishimura M, Kuno S, Ohta M. Fre quency of anti-AChR epsilon subunit-specific antibodies in MG. Autoimmunity 2003, 36: 151–154.
Leite MI, Waters P, Vincent A. Diagnostic use of autoantibodies in myasthenia gravis. Autoimmunity 2010, 43: 371–379.
Allen JA, Scala S, Jones HR. Ocular myasthenia gravis in a senior population: diagnosis, therapy, and prognosis. Muscle Nerve 2010, 41: 379–384.
Evoli A, Tonali PA, Padua L, Monaco ML, Scuderi F, Batocchi AP, et al. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain 2003, 126: 2304–2311.
Leite MI, Jacob S, Viegas S, Cossins J, Clover L, Morgan BP, et al. IgG1 antibodies to acetylcholine receptors in ’seronegative’ myasthenia gravis. Brain 2008, 131: 1940–1952.
Waters P, Jarius S, Littleton E, Leite MI, Jacob S, Gray B, et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch Neuro 2008, 65: 913–919.
Kupersmith MJ, Latkany R, Homel P. Development of generalized disease at 2 years in patients with ocular myasthenia gravis. Arch Neurol 2003, 60: 243–248.
Tzartos SJ, Barkas T, Cung MT, Mamalaki A, Marraud M, Orlewski P, et al. Anatomy of the antigenic structure of a large membrane autoantigen, the muscle-type nicotinic acetylcholine receptor. Immunol Rev 1998, 163: 89–120.
Sheng JR, Li LC, Prabhakar BS, Meriggioli MN. Acetylcholine receptor-alpha subunit expression in myasthenia gravis: a role for the autoantigen in pathogenesis? Muscle Nerve 2009, 40: 279–286.
Baggi F, Annoni A, Ubiali F, Milani M, Longhi R, Scaioli W, et al. Breakdown of tolerance to a self-peptide of acetylcholine receptor alpha-subunit induces experimental myasthenia gravis in rats. J Immunol 2004, 172: 2697–2703.
Lindstrom JM. Acetylcholine receptors and myasthenia. Muscle Nerve 2000, 23: 453–477.
Sun C, Meng F, Li Y, Jin Q, Li H, Li F. Antigen-specific immunoadsorption of anti-acetylcholine receptor antibodies from sera of patients with myasthenia gravis. Artif Cells Blood Substit Immobil Biotechnol 2010, 38: 99–102.
Kostelidou K, Trakas N, Tzartos SJ. Extracellular domains of the beta, gamma and epsilon subunits of the human acetylcholine receptor as immunoadsorbents for myasthenic autoantibodies: a combination of immunoadsorbents results in increased efficiency. J Neuroimmunol 2007, 190: 44–52.
Kaminski HJ, Kusner LL, Nash KV, Ruff RL. The gamma-subunit of the acetylcholine receptor is not expressed in the levator palpebrae superioris. Neurology 1995, 45: 516–518.
Kaminski HJ, Kusner LL, Block CH. Expression of acetylcholine receptor isoforms at extraocular muscle endplates. Invest Ophthalmol Vis Sci 1996, 37: 345–351.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Shi, QG., Wang, ZH., Ma, XW. et al. Clinical significance of detection of antibodies to fetal and adult acetylcholine receptors in myasthenia gravis. Neurosci. Bull. 28, 469–474 (2012). https://doi.org/10.1007/s12264-012-1256-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-012-1256-0