Abstract
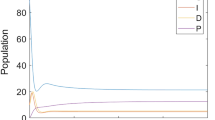
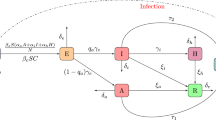
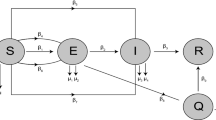
This paper deals with the following biological question: how influential is the environmental contamination on the transmission of EVD? Based on the works in (Bibby et al., Environ Sci Technol Lett 2:2–6, 2015; Leroy et al., Nature 438: 575–576, 2005; World Health Organization. Unprecedented number of medical staff infected with Ebola), we design a new mathematical model to address this question by assessing the effect of the Ebola virus contaminated environment on the dynamical transmission of EVD. The formulated model captures two infection pathways through both direct human-to-human transmission and indirect human-to-environment-to-human transmission by incorporating the environment as a transition and/or reservoir of Ebola viruses. We compute the basic reproduction number \({\mathcal {R}}^{env}_0\) for the model with environmental contamination and prove that the disease-free equilibrium is globally asymptotically stable (GAS) whenever \({\mathcal {R}}^{env}_0 \le 1\). When \({\mathcal {R}}^{env}_0 > 1\), we show that the said model has a unique endemic equilibrium which is GAS. Similar results hold for the free environmental contamination sub-model (without the incorporation of the indirect transmission). More precisely, for the latter model, calculate the corresponding basic reproduction number \({\mathcal {R}}^{h}_0\) and establish the GAS of the disease-free and endemic equilibria, whenever \({\mathcal {R}}^{h}_0 \le 1\) and \({\mathcal {R}}^{h}_0 > 1\), respectively. At the endemic level, we show that the number of infected individuals for the full model with the environmental contamination is greater than the corresponding number for the free environmental contamination sub-model. In conjunction with the inequality \({\mathcal {R}}^{h}_0 < {\mathcal {R}}^{env}_0\), our finding suggests a negative answer to the biological question under investigation, i.e. the contaminated environment plays a detrimental role on the transmission dynamics of EVD by increasing the endemic level and/or the severity of the outbreak. Therefore, it is natural to implement a control strategy which aim at reducing the severity of the disease by providing adequate hygienic living conditions, educate populations at risk to follow rigorously those basic hygienic conditions as well as ask them avoid contact with suspected contaminated objects. Further, we perform numerical simulations to support the theory.









Similar content being viewed by others
References
Agusto, F.B., Teboh-Ewungkem, M.I., Gumel, A.B.: Mathematical assessment of the effect of traditional beliefs and customs on the transmission dynamics of the 2014 Ebola outbreaks. BMC Med. 13, 96 (2015). doi:10.1186/s12916-015-0318-3
Althaus, C.: Estimating the reproduction number of Ebola (EBOV) during outbreak in West Africa. PLOS Curr. 1, Sept 2 (2014)
Anderson, R.M., May, R.M.: Infectious Diseases of Humans: Dynamics and Control. Oxford University Press, Oxford (1991)
Arata, A.A., Johnson, B.: Approaches toward studies on potential reservoirs of viral haemorrhagic fever in southern Sudan (1977). In: Pattyn, S.R.S. (ed.) Ebola Virus Haemorrhagic Fever, pp. 191–200 (1978)
Baize, S., Pannetier, D., Oestereich, L.: Emergence of Zaire Ebola virus disease in Guinea—preliminary report. New Engl. J Med. (2014)
Bani-Yabhoub, M., et al.: Reproduction numbers for infections with free-living pathogens growing in the environment. J. Biol. Dyn. 6(2), 923–940 (2012)
Bibby, K., et al.: Ebola virus persistence in the environment: state of the knowledge and research needs. Environ. Sci. Technol. Lett. 2, 2–6 (2015)
Blower, S.-M., Dowlatabadi, H.: Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. Int. Stat. Rev. 2, 229–243 (1994)
Bray, M.: Filoviridae. In: Richman, D.D., Whitley, R.J., Hayden, F.G. (eds.) Clinical Virology, p. 875. ASM Press, Washington, DC (2002)
Berge, T., Bowong, S., Lubuma, J. M.-S.: Global stability of a two-patch cholera model with fast and slow transmissions. Math. Comput. Simul. (2015), In Press
Berge, T., Moremedi, M.G., Lubuma, J.M.-S., Morris, N., Shava, R.K.: A simple mathematical for Ebola in Africa. Under review (2015)
Capasso, V., Paveri-Fontana, S.L.: A mathematical model for the 1973 cholera epidemic in the european mediterranean region. Revue dépidémioligié et de santé publiqué 27, 121 (1973)
Carr, J.: Applications Centre Manifold Theory. Springer, New York (1981)
Castillo-Chavez, C., Thieme, H.: Asymptotically autonomous epidemic models. In: Arino, O., Axelrod, D., Kimmel, M., Langlais, M. (eds.) Mathematical Population Dynamics: Analysis of Heterogeneity, p. 33. Springer, Berlin (1995)
Castillo-Chavez, C., Song, B.: Dynamical models of tuberculosis and their applications. Math. Biosci. Eng. 1(2), 361–404 (2004)
Chan, M.: Ebola Virus Disease in West Africa. http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/united-states-imported-case.html (Accessed on October 01, 2014)
Chitnis, N., Hyman, J.-M., Cushing, J.-M.: Determining important parameters in the spread of malaria through the sensitivity analysis of a mathematical model. Bull. Math. Biol. 70, 272–1-296 (2008). A no early end to the outbreak. New England J Med. September 25, (2014), doi:10.1056/NEJMp1409859
Chowell, G., Nishiura, N.: Transmission dynamics and control of Ebola virus disease (EVD): a review. BMC Med. 12, 196 (2014)
Chowell, G., et al.: The basic reproductive number of Ebola and the effects of public health measures: the cases of Congo and Uganda. J. Theor. Biol. 229, 119–126 (2004)
Codeço, C.T.: Endemic and epidemic dynamic of cholera: the role of the aquatic reservoir. BMC Infect. Dis. 1, 1
Diekmann, O., Heesterbeek, J.A.P., Roberts, M.G.: The construction of next-generation matrices for compartmental epidemic models. J. R. Soc. Interface 7, 873–885 (2010)
Ebola virus disease. Fact sheet \(N^{0}\) 103, Updated April 2014, http://www.who.int/mediacentre/factsheets/fs103/en/
Eichner, M., Dowell, S.F., Firese, N.: Incubation period of Ebola Hemorrhagic virus subtype Zaire. Public Health Res. Perspect. 2, 1 (2011)
Fasina, F. O., Shittu, A., Lazarus, D., Tomori, O., Simonsen, L., Viboud, C., Chowell, G.: Transmission dynamics and control of Ebola virus disease outbreak in Nigeria, July to September 2014. Euro Surveill. 2014;19(40). Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20920
Feldmann, H.: Ebola—a growing threat? New Engl. J. Med. (2014)
Feldmann, H., et al.: Ebola virus ecology: a continuing mystery. Trends Microbiol. 12, 433–437 (2004)
Formenty, P., Hatz, C., Le Guenno, B., et al.: Human infection due to Ebola virus, subtype Cte d’Ivoire: clinical and biologic presentation. J. Infect Dis. 179(Suppl 1), S48 (1999)
Groseth, A., Feldmann, H., Strong, J.E.: The ecology of Ebola Virus. J. Infect Dis. 15(9), 408–416 (2007)
Ivorra, B., Ngom, D., Ramos, A.M.: Be-CoDiS: a mathematical model to predict the risk of human diseases spread between countries-validation and application to the 2014–2015 ebola virus disease epidemic. Bull. Math. Biol. 77, 1668–1704 (2015). doi:10.1007/s11538-015-0100-x
Jahrling, P.B., Geisbert, T.W., Dalgard, D.W., et al.: Preliminary report: isolation of Ebola virus from monkeys imported to USA. Lancet 335, 502 (1990)
Kuniholm, M.H.: Bat exposure is a risk factor for Ebola virus infection. In: Filoviruses: Recent Advances and Future Challenges: An ICID Global Symposium (2006)
Lakshmikantham, L., Leela, S., Martynyuk, A.A.: Stability Analysis of Nonlinear Systems, pp. 155–170. Marcel Dekker, Inc, New York (1989)
Legrand, J., Grais, R.F., Boelle, P.Y., Valleron, A.J., Flahault, A.: Understanding the dynamics of Ebola epidemics. Epidemiol. Infect. 135, 610–621 (2007)
Lekone, P.E., Finkenstädt, B.F.: Statistical inference in a stochastic epidemic SEIR model with control intervention: Ebola as a case study. Epidemiol. Infect. 62, 1170–1177 (2006)
Leroy, E.M., et al.: Fruit bats as reservoirs of Ebola virus. Nature 438, 575–576 (2005)
Leroy, E.M., et al.: Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science 303(5656), 387–390 (2004)
Li, M.Y., Graef, J.R., Wang, L., Karsai, J.: Global dynamics of a SEIR model with varying total population size. Math. Biosci. 160, 191–213 (1999)
Li, M.Y., Muldowney, J.S.: A geometrical approach to global-stability problems. Math. Biosci. 27, 1070–1083 (1996)
Marino, S., Hogue, I.-B., Ray, C.-J., Kirschner, D.-E.: A methodology for performing global uncertainty and sensitivity analysis in systems biology. J. Theor. Biol. 254, 178–196 (2008)
Miranda, M.E., Ksiazek, T.G., Retuya, T.J., et al.: Epidemiology of Ebola (subtype Reston) virus in the Philippines, 1996. J. Infect. Dis. 179(Suppl 1), S115 (1999)
Ndanguza, D., et al.: Statistical data analysis of the 1995 Ebola outbreak in the Democratic Republic of Congo. J. Infect. Dis. 24, 55–68 (2013)
Oähea, T.J., et al.: Bat flight and zoonotic viruses. J. Infect. Dis. 20, 5 (2014)
Onyango, C.O., Opoka, M.L., Ksiazek, T.G., et al.: Laboratory diagnosis of Ebola hemorrhagic fever during an outbreak in Yambio, Sudan, 2004. J. Infect. Dis. 196(Suppl 2), S193 (2007)
Piercy, T.J., et al.: The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J. Appl. Microbiol. 109(5), 1531–1539 (2010)
Pourrut, X., et al.: Spatial and temporal patterns of Zaire ebola virus antibody prevalence in the possible reservoir bat species. J. Infect. Dis. 15; 196(Suppl 2), S176–A183 (2007)
Sanchez, A., Lukwiya, M., Bausch, D., et al.: Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: cellular responses, virus load, and nitric oxide levels. J. Virol. 78, 10370 (2004)
Smith, H.L., Waltman, P.: The Theory of the Chemostat. Cambridge University, Cambridge (1995)
The Centers for Disease Control and Prevention. First imported case of Ebola diagnosed in the United States
The Centers for Disease Control and Prevention. 2014 Ebola outbreak in West Africa. http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/index.html (Accessed on September 29, 2014)
Towers, S., Patterson-Lomba, O., Castillo-Chavez, C.: Temporal variations in the effective reproduction number of the 2014 West Africa Ebola outbreak, PLOS Currents Outbreaks, Sept 18, (2014)
Towner, J.S., Sealy, T.K., Khristova, M.L., et al.: Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathogens 4, e1000212 (2008)
van Den Driessche, P., Watmough, J.: Reproduction numbers and sub-threshold endemic equilibria for compartmental models of desease transmission. Math. Biosci. 180, 29–48 (2002)
World Health Organization. Ebola response roadmap situation report, 26 September 2014. http://apps.who.int/iris/bitstream/10665/135029/1/roadmapupdate26sept14-eng.pdf?ua=1 (Accessed on September 26, 2014)
World Health Organization. Ebola situation in Senegal remains stable. http://www.who.int/mediacentre/news/ebola/12-september-2014/en/ (Accessed on September 12, 2014)
World Health Organization. Unprecedented number of medical staff infected with Ebola. http://www.who.int/mediacentre/news/ebola/25-august-2014/en/ (Accessed on August 25, 2014)
WHO Ebola Response Team. Ebola Virus Disease in West Africa - TheFirst 9 Months of the Epidemic and Forward Projections. N. Engl. J.Med. (2014)
World Health Organization. Global Alert Response. Ebola virus disease—Democratic Republic of Congo. http://www.who.int/csr/don/$2014_08_27_.$ebola/en/ (Accessed on August 28, 2014). doi:10.1086/674795
Youkee, D. et al.: Assessment of environmental contamination and environmental decontamination practices within an ebola holding unit, Freetown, Sierra Leone. PLOS ONE, December 1, 2015. doi:10.137/journal.pone.0145167
Zhang, Z., Ma, Z.: Global dynamics of an SEIR epidemic model with saturating contact rate. Math. Biosci. 185, 15–32 (2003)
Acknowledgments
The first (T.B.) and the third (J.L.) authors are grateful to the South African Research Chairs Initiative (SARChI Chair), in Mathematical Models and Methods in Bioengineering and Biosciences. The first (T.B.) and the second (S.B.) authors acknowledge the support of Center of Excellence Cameroon (CETIC). The authors are also grateful to the three anonymous referees and to Prof Chin-Hong Park, Editor in Chief of this journal, whose, suggestions and remarks substantially improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix: Proof of Theorem 3.8
Appendix: Proof of Theorem 3.8
To establish Theorem 3.8 when \({\mathcal {R}}^{env}_0 >1\) which shows the local stability of subsystem (2.9), we used the center manifold theory proposed in [15]. To this end, we introduce the following change of variables: \(x_1=S_h,\, x_2= E_h, \, x_3= I_h, \, x_4= R_h,\, x_5 = V\). Therefore, \(x'_1 = S'_h, \, x'_2 = E'_h, \,x'_3 = I'_h, \,x'_4 = R'_h, \,x'_5 = V'\). The disease-free equilibrium of subsystem (2.9) becomes \(x^*_0 = ({\varLambda }_h/\mu _h,0,0,0,0)\).
Let \(\sigma _v > 0\) be the non-negative real numbers such that \(\beta _{hv}=\sigma _v\beta _{hh}\), , then the basic reproduction number \({\mathcal {R}}^{env}_0\) becomes
Let \(\beta _{hh}=\phi \) be the bifurcation parameter, then solving the equation \({\mathcal {R}}^{env}_0 =1\) for \(\beta _{hh}\) yields
Notice that \({\mathcal {R}}^{env}_0 > 1\) if and only if \(\beta _{hh} > \beta ^*_{hh}\).
With these notations, system (2.9) takes the form:
The Jacobian matrix of subsystem (6.1) at the disease-free equilibrium \(x^*_0\) when \(\phi = \phi ^*\) is
It is straightforward that the transformed system (6.1), with \(\phi =\phi ^*\) has a hyperbolic equilibrium point (i.e., the Jacobian matrix \(J_{\phi ^*}\) has a simple eigenvalue with zero real part (here, zero is a simple eigenvalue), and the remaining eigenvalues have negative real parts). Therefore the Center Manifold Theory [15] can applied to analyze the dynamics of subsystem (6.1) near the bifurcation parameter \(\phi =\phi ^*\). It is easy to see that a corresponding right-eigenvector of \(J_{\phi ^*}\) associated to the zero eigenvalue is \(\mathbf{w} = (w_1, w_2,w_3,w_4,w_5)^T\) , and a corresponding non-negative left-eigenvector associated to zero is given by \(\mathbf{v} = (v_1, v_2, v_3, v_4, v_5)\), where
To apply Theorem 4.1 in [15] and determine the nature and the direction of the bifurcation at \({\mathcal {R}}^{env}_0 = 1\), we must compute the following quantities:
The only non-vanishing second partial derivatives of f corresponding to the non-zero components of \(\mathbf v\) evaluated at \(\left( x_0^*,\phi ^*\right) \) are:
Thus,
Thanks to item 4 of Theorem 4.1 in [15], the endemic \(P^*_h\) of sub-model (2.9) is locally asymptotically stable when \({\mathcal {R}}^{env}_0>1\), but near to 1. Moreover, the bifurcation of the subsystem (2.9) around \({\mathcal {R}}^{env}_0=1\) is trans-critical. The proof is complete.
Rights and permissions
About this article
Cite this article
Tsanou, B., Bowong, S., Lubuma, J. et al. Assessing the impact of the environmental contamination on the transmission of Ebola virus disease (EVD). J. Appl. Math. Comput. 55, 205–243 (2017). https://doi.org/10.1007/s12190-016-1033-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12190-016-1033-8