Abstract
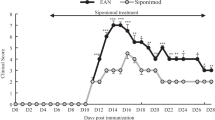
Experimental autoimmune neuritis (EAN) serves as an animal model for human Gullain–Barre syndrome (GBS), an autoimmune disease causing demyelination and inflammation of peripheral nerves. Macrophages, which play a major role in this autoimmune inflammatory process, can be selectively targeted by high doses of bisphophonates. The goal of this study was to examine the effect of the bisphosphonate, clodronate, on the severity of the EAN model. EAN was induced in female adult rats by immunization with bovine peripheral myelin. A number of treatment protocols with clodronate were used based on the common dosage regimen of 20 mg/kg in humans starting with the appearance of clinical signs on day 10 post-immunization. The clinical parameters measured included a clinical score, a motor performance test performed on a Rotarod and body weight. The expression of the matrix metaloprotease (MMP-9) in the sciatic nerves was measured as a marker of inflammatory macrophages. Treatment with clodronate, 20 mg/kg daily and 40 mg/kg every 2 days, significantly reduced the disease severity (a 75 % decrease in severity, p < 0.01 by ANOVA) as measured by the clinical score compared to controls. Performance on the Rotarod test and body weight confirmed the clinical score findings. MMP-9 expression levels were significantly lower in the sciatic nerves of clodronate-treated rats. The present findings support the efficiency of clodronate in inflammatory diseases of the peripheral nervous system. The mechanism of action includes inhibition of inflammatory macrophages. The results suggest the use of bisphosphonates be considered in humans with GBS.


Similar content being viewed by others
References
Soffer D, Feldman S, Alter M. Epidemiology of Guillain-Barre syndrome. Neurology. 1978;28:686–90.
Kamm C, Zettl UK. Autoimmune disorders affecting both the central and peripheral nervous system. Autoimmun Rev. 2012;11:196–202.
Kieseier BC, Lehmann HC, Horste GM. Autoimmune diseases of the peripheral nervous system. Autoimmun Rev. 2012;11:191–5.
Israeli E, Agmon-Levin N, Blank M, Chapman J, Shoenfeld Y. Guillain-Barre syndrome–a classical autoimmune disease triggered by infection or vaccination. Clin Rev Allergy Immunol. 2012;42:121–30.
Shoenfeld Y, George J, Peter J. Guillain-Barre as an autoimmune disease. Int Arch Allergy Immunol. 1996;109:318–26.
Nussinovitch U, Shoenfeld Y. The role of gender and organ specific autoimmunity. Autoimmun Rev. 2012;11:A377–85.
Kadlubowski M, Hughes R. Identification of the neuritogen for experimental allergic neuritis. Nature. 1979;277:140–1.
Shin H, McFarlane E, Pollard J, Watson E. Induction of experimental allergic neuritis with synthetic peptides from myelin P2 protein. Neurosci Lett. 1989;102:309–12.
Waksman N, Adams R. Allergic neuritis: an experimental disease of rabbits induced by the injection of peripheral nervous tissue and adjuvant. J Exp Med. 1955;102:213–25.
Aronovich R, Katzav A, Chapman J. The strategies used for treatment of experimental autoimmune neuritis (EAN): a beneficial effect of glatiramer acetate administered intraperitoneally. Clin Rev Allergy Immunol. 2012;42:181–8.
Kafri M, Drory V, Wang N, Rabinowitz R, Korczyn A, Chapman J. Assessment of experimental autoimmune neuritis in the rat by electrophysiology of the tail nerve. Muscle Nerve. 2002;25:51–7.
Delmas PD. Treatment of postmenopausal osteoporosis. Lancet. 2002;359:2018–26.
Ghinoi V, Brandi ML. Clodronate: mechanisms of action on bone remodelling and clinical use in osteometabolic disorders. Expert Opin Pharmacother. 2002;3:1643–56.
Prestwood KM, Raisz LG. Prevention and treatment of osteoporosis. Clin Cornerstone. 2002;4:31–41.
Schimmer RC, Bauss F. Effect of daily and intermittent use of ibandronate on bone mass and bone turnover in postmenopausal osteoporosis: a review of three phase II studies. Clin Ther. 2003;25:19–34.
Benford HL, McGowan NW, Helfrich MH, Nuttall ME, Rogers MJ. Visualization of bisphosphonate-induced caspase-3 activity in apoptotic osteoclasts in vitro. Bone. 2001;28:465–73.
Frith JC, Monkkonen J, Auriola S, Monkkonen H, Rogers MJ. The molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate: evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosis. Arthritis Rheum. 2001;44:2201–10.
Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995;10:1478–87.
Selander KS, Monkkonen J, Karhukorpi EK, Harkonen P, Hannuniemi R, Vaananen HK. Characteristics of clodronate-induced apoptosis in osteoclasts and macrophages. Mol Pharmacol. 1996;50:1127–38.
Comi C, Fleetwood T, Dianzani U. The role of T cell apoptosis in nervous system autoimmunity. Autoimmun Rev. 2012;12(2):150–6.
Teitelbaum SL, Tondravi MM, Ross FP. Osteoclasts, macrophages, and the molecular mechanisms of bone resorption. J Leukoc Biol. 1997;61:381–8.
Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, Martin TJ, Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA. 1990;87:7260–4.
Mian M, Benetti D, Aloisi R, Rosini S, Fantozzi R. Effects of bisphosphonate derivatives on macrophage function. Pharmacology. 1994;49:336–42.
Makkonen N, Hirvonen MR, Teravainen T, Savolainen K, Monkkonen J. Different effects of three bisphosphonates on nitric oxide production by RAW 264 macrophage-like cells in vitro. J Pharmacol Exp Ther. 1996;277:1097–102.
Huitinga I, van Rooijen N, de Groot CJ, Uitdehaag BM, Dijkstra CD. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J Exp Med. 1990;172:1025–33.
Huitinga I, Ruuls SR, Jung S, Van Rooijen N, Hartung HP, Dijkstra CD. Macrophages in T cell line-mediated, demyelinating, and chronic relapsing experimental autoimmune encephalomyelitis in Lewis rats. Clin Exp Immunol. 1995;100:344–51.
Tran EH, Hoekstra K, van Rooijen N, Dijkstra CD, Owens T. Immune invasion of the central nervous system parenchyma and experimental allergic encephalomyelitis, but not leukocyte extravasation from blood, are prevented in macrophage-depleted mice. J Immunol. 1998;161:3767–75.
Jung S, Huitinga I, Schmidt B, Zielasek J, Dijkstra CD, Toyka KV, Hartung HP. Selective elimination of macrophages by dichlormethylene diphosphonate-containing liposomes suppresses experimental autoimmune neuritis. J Neurol Sci. 1993;119:195–202.
Kadlubowski M, Hughes R, Gregson N. Experimental allergic neuritis in the Lewis rat: characterization of the activity of peripheral myelin and its major basic protein P2. Brain Res. 1980;184:439–54.
McDermott JR, Keith AB. Antigen-induced suppression of experimental allergic neuritis in the guinea pig. J Neurol Sci. 1980;46:137–43.
Fux M, van Rooijen N, Owens T. Macrophage-independent T cell infiltration to the site of injury-induced brain inflammation. J Neuroimmunol. 2008;203:64–72.
Polfliet MM, van de Veerdonk F, Dopp EA, van Kesteren-Hendrikx EM, van Rooijen N, Dijkstra CD, van den Berg TK. The role of perivascular and meningeal macrophages in experimental allergic encephalomyelitis. J Neuroimmunol. 2002;122:1–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katzav, A., Bina, H., Aronovich, R. et al. Treatment for experimental autoimmune neuritis with clodronate (Bonefos). Immunol Res 56, 334–340 (2013). https://doi.org/10.1007/s12026-013-8406-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-013-8406-y