Abstract
Pediatric gastroesophageal reflux disease (GERD) is common in infants and children; diagnosis is often based on symptom presentation. This paper reviews psychometric characteristics and approaches to validation of currently available pediatric GERD questionnaires. Patient-reported outcomes allow disease and treatment to be characterized in meaningful ways to patients and clinicians. Outcome measures must demonstrate reliability and validity for use in practice and in clinical trials. Reliability assesses the consistency of measures, whereas validity examines whether the instrument measures what it is purported to measure. Development of questionnaires for use with children also requires consideration regarding the respondent. Measures of pediatric GERD symptoms include the GERQ, GERQ-R, and GSQ. These measures have advantages and disadvantages with regard to feasibility, reliability, and validity. Questionnaires are lacking for children older than 4 years.
Similar content being viewed by others
References and Recommended Reading
Gold BD: Outcomes of pediatric gastroesophageal reflux disease: in the first year of life, in childhood, and in adults⋯oh, and should we really leave helicobactor pylori alone? J Pediatr Gastroenterol Nutr 2003, 37:S33-S39.
Gold BD: Review article: Epidemiology and management of gastro-oesophageal reflux in children. Aliment Pharmacol Ther 2004, 19(Suppl 1):22–27.
Tolia V, Ferry G, Gunasekaran T, et al.: Efficacy of lansoprazole in the treatment of gastroesophageal reflux disease in children. J Pediatr Gastroenterol Nutr 2002, 35:S308-S318.
Hassal E, Israel D, Shepherd R, et al.: Omeprazole for treatment of chronic erosive esophagitis in children: a multicenter study of efficacy, safety, tolerability and dose requirements. International Pediatric Omeprazole Study Group. J Pediatr 2000, 137:800–807.
Rudolph CD, Mazur LJ, Lipak GS, et al.: Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr 2001, 32(Suppl 2):S1-S31.
Vandenplas Y, Belli D, Benhamou P, et al.: A critical appraisal of current management practices for infant regurgitation: recommendations of a working party. Eur J Pediatr. 1997, 156:343–357.
Botstein P: Needs and new policies for medicines for children: the FDA, United States incentives, and international doings. Drug Inform J 2000, 34:203–205.
Kleist P: Pediatric drug development. Appl Clin Trials 2002, 40–48.
Scientific Advisory Committee of the Medical Outcomes Trust: Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res 2002, 11:193–205. This article reviews eight key attributes of patient-reported outcome instruments, including conceptual and measurement model, reliability, validity, responsiveness, interpretability, respondent and administrative burden, alternate forms, and cultural and language adaptations. It also discusses what is necessary for identification of a well-developed instrument.
Fayers P, Hays RD: Assessing Quality of Life in Clinical Trials, edn 2. New York: Oxford University Press; 2005.
McDowell I, Newell C: Measuring Health: A Guide to Rating Scales and Questionnaires, edn 2. New York: Oxford University Press; 1996.
Hays RD, Revicki DA: Reliability and validity including responsiveness. In Assessing Quality of Life in Clinical Trials, edn 2. Edited by Fayers P, Hays RD. New York: Oxford University Press; 2005.
Streiner DL, Norman GR: Health Measurement Scales: A Practical Guide to Their Development and Use, edn 2. New York: Oxford University Press; 1995.
Revicki DA, Osoba D, Fairclough D, et al.: Recommendations on health-related quality of life research to support labeling and promotional claims in the United States. Quality Life Res 2000, 9:887–900.
Cronbach LJ: Coefficient alpha and the internal structure of tests. Psychometrika 1951, 16:297–334.
Nunnally JC, Bernstein IH: Psychometric Theory, edn 3. New York: McGraw-Hill; 1994.
Orenstein SR, Cohn JF, Shalaby TM, Kartan R: Reliability and validity of an infant gastroesophageal reflux questionnaire. Clin Pediatr 1993, 32:472–484.
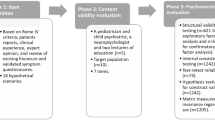
Kleinman L, Rothman M, Strauss R, et al.: The infant gastroesophageal reflux questionnaire revised: development and validation as an evaluative instrument. Clin Gastroenterol Hepatol, 2006, in press. This article provides validation information on the IGERQ-R, a 12-item diagnostic and evaluative infant GERD symptom questionnaire designed to measure changes in symptoms over time. It was validated in an international study using verified translations and demonstrated significant differences between healthy control subjects and infants with GERD. The questionnaire is published as an appendix.
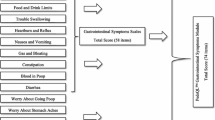
Deal L, Gold BD, Gremse DA, et al.: Age-specific questionnaires distinguish GERD symptom frequency in infants and young children: development and initial validation. J Pediatr Gastroenterol Nutr 2005, 41:178–185. This article discusses the most recently published pediatric GERD symptom questionnaire. It provides initial validation information on two versions of a pediatric GERD symptom questionnaire designed to measure frequency and severity of symptoms in infants and younger children. The questionnaire demonstrates significant differences in score between healthy children and those with pediatric GERD.
Matza LS, Swensen AR, Flood EM, et al.: Assessment of health-related quality of life in children: a review of conceptual, methodological and regulatory issues. Value Health 2004, 7:79–92. There are unique challenges to assessing patient-reported outcomes in children. This article addresses recent pediatric regulatory developments, issues in defining health-related quality of life in children, measurement issues, available questionnaires, and recommendations for additional research in the psychiatric field.
Eiser C, Morse R: Can parents rate their child’s healthrelated quality of life? Results of systematic review. Qual Life Res 2001, 10:347–357.
Levi RB, Drotar D: Health-related quality of life in childhood cancer: discrepancy in parent-child reports. Int J Cancer Suppl. 1999, 12:58–64.
Riley AW: Evidence that school-age children can self-report on their health. Ambul Pediatr 2004, 4(Suppl):371–376.
Rebok G, Riley A, Forrest C, et al.: Elementary school-aged children’s reports of their health: a cognitive interviewing study. Quality of Life Research. 2001, 10:59–70.
Orenstein SR, Shalaby TM, Cohn JF: Reflux symptoms in 100 normal infants: diagnostic validity of the infant gastroesophageal reflux questionnaire. Clin Pediatr (Phila) 1996, 35:607–614.
Nelson SP, Chen EH, Syniar GM, Christoffel K:. Prevalence of symptoms of gastroesophageal reflux during infancy: a pediatric practice-based study. Arch Pediatr Adoles Med 1997, 151:569–572.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kleinman, L., Revicki, D.A. & Flood, E. Validation issues in questionnaires for diagnosis and monitoring of gastroesophageal reflux disease in children. Curr Gastroenterol Rep 8, 230–236 (2006). https://doi.org/10.1007/s11894-006-0080-y
Issue Date:
DOI: https://doi.org/10.1007/s11894-006-0080-y