Summary
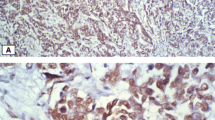
Forkhead Box c2 (FOXC2) is a member of forkhead/winged-helix family of transcription factors. The relationship between FOXC2 and invasive breast cancers, including basal-like breast cancer (BLBC, a subtype of breast cancer), remains to be elucidated. In this study, immunohistochemistry was used to detect the expression of FOXC2 in samples from 103 cases of invasive breast cancers and 15 cases of normal mammary glands. The relationship between FOXC2 and clinical parameters of invasive breast cancers such as patient’s age, tumor size, lymph node metastasis, tumor grade, the expression of ER, PR, HER-2 and p53, and Ki-67 labeling index (LI) was evaluated. The expression of FOXC2 was detected in parent MCF7 cells, MCF cells transfected with FOXC2 expression vectors and MDA-MB-435 cells by immunohistochemistry and Western blotting. Transwell assay was used to determine the invasive ability of these cells. The results showed that FOXC2 was strongly expressed in basal epithelial cells in normal mammary glands and weakly expressed or even not expressed in glandular epithelial cells. The majority of invasive breast cancers (71.8%, 74/103) had negative or weak expression of FOXC2. However, FOXC2 was strongly expressed in 60.7% of BLBCs. Moreover, FOXC2 was related with tumor grade, p53 expression, ki-67 LI and lymph nodes metastasis. It was expressed in FOXC2-transfected MCF cells and MDA-MB-435 cells but not in parent MCF cells. Transwell assay revealed that MCF cells transfected with FOXC2 expression vectors were more aggressive than the parent MCF cells, suggesting a positive correlation between FOXC2 and the invasion of breast cancer. It was concluded that there is a significant association between FOXC2 and the metastasis of invasive breast cancer. FOXC2 may be used as a new marker for the diagnosis and prognosis prediction of different subtypes of invasive breast cancers.
Similar content being viewed by others
References
Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer, 2010,127(12):2893–2917
Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin, 2011,61(2):69–90
Fang J, Dagenais SL, Erickson RP, et al. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am J Hum Genet, 2000,67(6):1382–1388
Cederberg A, Gronning LM, Ahren B, et al. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell, 2001,106(5):563–573
De Val S, Chi NC, Meadows SM, et al. Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell, 2008,35(6):1053–1064
Xue Y, Cao R, Nilsson D, et al. FOXC2 controls Ang-2 expression and modulates angiogenesis, vascular patterning, remodeling, and functions in adipose tissue. Proc Natl Acad Sci USA, 2008,105(29):10167–10172
Sano H, Leboeuf JP, Novitskiy SV, et al. The Foxc2 transcription factor regulates tumor angiogenesis. Biochem Biophys Res Commun, 2010,392(2):201–206
Watanabe T, Kobunai T, Yamamoto Y, et al. Gene expression of mesenchyme forkhead 1 (FOXC2) significantly correlates with the degree of lymph node metastasis in colorectal cancer. Int Surg, 2011,96(3):207–216
Jiang W, Pang XG, Wang Q, et al. Prognostic role of Twist, Slug, and Foxc2 expression in stage I non-small-cell lung cancer after curative resection. Clin Lung Cancer, 2012, 13(4):280–287
Nishida N, Mimori K, Yokobori T, et al. FOXC2 is a novel prognostic factor in human esophageal squamous cell carcinoma. Ann Surg Oncol, 2011,18(2):535–542
Mani SA, Yang J, Brooks M, et al. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci USA, 2007,104(24):10069–10074
Li Y, Yang W, Yang Q, et al. Nuclear localization of GLI1 and elevated expression of FOXC2 in breast cancer is associated with the basal-like phenotype. Histol Histopathol, 2012,27(4):475–484
Omoteyama K, Mikami Y, Takagi M. Foxc2 induces expression of MyoD and differentiation of the mouse myoblast cell line C2C12. Biochem Biophys Res Commun, 2007,358(3):885–889
Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature, 2000,406(6797):747–752
Rakha EA, Reis-Filho JS, Ellis IO. Impact of basal-like breast carcinoma determination for a more specific therapy. Pathobiology, 2008,75(2):95–103
Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA, 2001,98(19):10869–10874
Shimeld SM, Degnan B, Luke GN. Evolutionary genomics of the Fox genes: origin of gene families and the ancestry of gene clusters. Genomics, 2010,95(5):256–260
Tsuda H, Takarabe T, Hasegawa T, et al. Myoepithelial differentiation in high-grade invasive ductal carcinomas with large central acellular zones. Hum Pathol, 1999, 30(10):1134–1139
Korsching E, Packeisen J, Liedtke C, et al. The origin of vimentin expression in invasive breast cancer: epithelial-mesenchymal transition, myoepithelial histogenesis or histogenesis from progenitor cells with bilinear differentiation potential? J Pathol, 2005,206(4):451–457
Soussi T, Beroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat Rev Cancer, 2001,1(3):233–240
Arjonen A, Kaukonen R, Mattila E, et al. Mutant p53-associated myosin-X upregulation promotes breast cancer invasion and metastasis. J Clin Invest, 2014,124(3):1069–1082
Braicu C, Pileczki V, Irimie A, et al. p53siRNA therapy reduces cell proliferation, migration and induces apoptosis in triple negative breast cancer cells. Mol Cell Biochem, 2013,381(1–2):61–68
Bidard FC, Matthieu MC, Chollet P, et al. p53 status and efficacy of primary anthracyclines/alkylating agent-based regimen according to breast cancer molecular classes. Ann Oncol, 2008,19(7):1261–1255
de Azambuja E, Cardoso F, de Castro GJ, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer, 2007,96(10):1504–1513
Blick T, Widodo E, Hugo H, et al. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis, 2008,25(6):629–642
Kargozaran H, Yuan SY, Breslin JW, et al. A role for endothelial-derived matrix metalloproteinase-2 in breast cancer cell transmigration across the endothelial-basement membrane barrier. Clin Exp Metastasis, 2007,24(7):495–502
Wu ZS, Wu Q, Yang JH, et al. Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int J Cancer, 2008,122(9):2050–2056
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was supported by a grant from the National Natural Science Foundation of China (No. 30570725).
Rights and permissions
About this article
Cite this article
Dai, J., Wang, Jy., Yang, Ll. et al. Correlation of Forkhead Box c2 with subtypes and invasive ability of invasive breast cancer. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 34, 896–901 (2014). https://doi.org/10.1007/s11596-014-1370-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-014-1370-5