Abstract
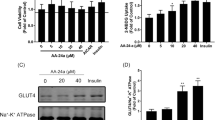
Flos Magnoliae (FM) is a commonly used Chinese medicinal herb for symptomatic relief of allergic rhinitis, sinusitis and headache. Although several FM species have been used as substitutes or adulterants for clinical use, possible differences in their pharmacological actions have not been reported. To confirm the effects of FM on skeletal muscle glucose metabolism, we tested the effects of several compounds isolated from FM on glucose uptake by L6 myotubes. We found that fargesin, a component of FM, dose-dependently stimulated glucose consumption in L6 myotubes, which was accompanied by enhanced glucose transporter (GLUT)-4 translocation to the cell surface. Fargesin-stimulated glucose uptake was blocked by wortmannin, a phosphatidylinositol-3 kinase (PI3 K) inhibitor. Fargesin stimulated Akt phosphorylation, a key component in the insulin signaling pathway, which was completely inhibited by wortmannin. Here, we demonstrated that fargesin, a bioactive component of Flos Magnoliae, increases basal glucose uptake and GLUT4 translocation in L6 myotubes by activating the PI3 K–Akt pathway.




Similar content being viewed by others
References
Zaid H, Antonescu CN, Randhawa VK, Klip A (2008) Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem J 413:201–215
Smith AG, Muscat GE (2005) Skeletal muscle and nuclear hormone receptors: implications for cardiovascular and metabolic disease. Int J Biochem Cell Biol 37:2047–2063
DeFronzon RA, Gunnarsson R, Bjorkman O, Olsson M, Wahren J (1985) Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest 76:149–155
Musi N, Hayashi T, Fujii N, Hirshman MF, Witters LA, Goodyear LJ (2001) AMP-activated protein kinase activity and glucose uptake in rat skeletal muscle. Am J Physiol Endocrinol Metab 280:E677–E684
Zheng D, MacLean PS, Pohnert SC, Knight JB, Olson AL, Winder WW, Dohm GL (2001) Regulation of muscle GLUT-4 transcription by AMP-activated protein kinase. J Appl Physiol 91:1073–1083
Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799–806
Björnholm M, Kawano Y, Lehtihet M, Zierath JR (1997) Insulin receptor substrate-1 phosphorylation and phosphatidylinositol 3-kinase activity in skeletal muscle from NIDDM subjects after in vivo insulin stimulation. Diabetes 46:524–527
Dohm GL, Tapscott EB, Pories WJ, Dabbs DJ, Flickinger EG, Meelheim D, Fushiki T, Atkinson SM, Elton CW, Caro JF (1998) An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J Clin Invest 82:486–494
Towler MC, Hardie DG (2007) AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100:328–341
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174
Konrad D, Rudich A, Bilan PJ, Patel N, Richardson C, Witters LA, Klip A (2005) Troglitazone causes acute mitochondrial membrane depolarisation and an AMPK-mediated increase in glucose phosphorylation in muscle cells. Diabetologia 48:954–966
Hwang JT, Kwon DY, Yoon SH (2009) AMP-activated protein kinase: a potential target for the diseases prevention by natural occurring polyphenols. N Biotechnol 26:17–22
Shen Y, Li CG, Zhou SF, Pang EC, Story DF, Xue CC (2008) Chemistry and bioactivity of Flos Magnoliae, a Chinese herb for rhinitis and sinusitis. Curr Med Chem 15:1616–1627
Kobayashi S, Kimura I, Kimura M (1996) Inhibitory effect of magnosalin derived from Flos magnoliae on tube formation of rat vascular endothelial cells during the angiogenic process. Biol Pharm Bull 19:1304–1306
Kim EK, Song MY, Kim IS, Moon WS, Ryu DG, So HS, Park R, Park JW, Kwon KB, Park BH (2008) Beneficial effect of Flos magnoliae extract on multiple low dose streptozotocin-induced type 1 diabetes development and cytokine-induced beta-cell damage. Int J Mol Med 22:481–488
Ahmed AA, Mahmoud AA, Ali ET, Tzakou O, Couladis M, Mabry TJ, Gáti T, Tóth G (2002) Two highly oxygenated eudesmanes and 10 lignans from Achillea holosericea. Phytochemistry 59:851–856
Mitsuo M, Hiroyuki K, Hiromu K (1994) Microbial oxidation of (+)-epimagnolin a by Aspergillus niger. Phytochemistry 35:1191–1193
Biavatti MW, Vieira PC, da Silva MF, Fernandes JB, Degani AL, Cass QB, Schefer AB, Ferreira AG (2001) Separation and NMR studies on lignans of Raulinoa echinata. Phytochem Anal 12:64–68
Mitsumoto Y, Burdett E, Grant A, Klip A (1991) Differential expression of the GLUT1 and GLUT4 glucose transporters during differentiation of L6 muscle cells. Biochem Biophys Res Commun 175:652–659
Ziel FH, Venkatesan N, Davidson NB (1998) Glucose transport is rate limiting for skeletal muscle glucose metabolism in normal and STZ-induced diabetic rats. Diabetes 37:885–890
Holmes BF, Kurth-Kraczek EJ, Winder WW (1999) Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol 87:1990–1995
Farese RV, Sajan MP, Standaert ML (2005) Insulin-sensitive protein kinases (atypical protein kinase C and protein kinase B/Akt): actions and defects in obesity and type II diabetes. Exp Biol Med (Maywood) 230:593–605
Guo T, Deng YX, Xie H, Yao CY, Cai CC, Pan SL, Wang YL (2011) Antinociceptive and anti-inflammatory activities of ethyl acetate fraction from Zanthoxylum armatum in mice. Fitoterapia 82:347–351
Lim H, Son KH, Bae KH, Hung TM, Kim YS, Kim HP (2009) 5-Lipoxygenase-inhibitory constituents from Schizandra fructus and Magnolia flos. Phytother Res 23:1489–1492
Kim JS, Kim JY, Lee HJ, Lim HJ, Lee DY, Kim DH, Ryu JH (2010) Suppression of inducible nitric oxide synthase expression by furfuran lignans from flower buds of Magnolia fargesii in BV-2 microglial cells. Phytother Res 24:748–753
Chae SH, Kim PS, Cho JY, Park JS, Lee JH, Yoo ES, Baik KU, Lee JS, Park MH (1998) Isolation and identification of inhibitory compounds on TNF-alpha production from Magnolia fargesii. Arch Pharm Res 21:67–69
Watson RT, Pessin JE (2007) GLUT4 translocation: the last 200 nanometers. Cell Signal 19:2209–2217
Acknowledgments
This study was performed at a laboratory supported by an endowment from Erina Co., Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, SS., Cha, BY., Choi, BK. et al. Fargesin, a component of Flos Magnoliae, stimulates glucose uptake in L6 myotubes. J Nat Med 67, 320–326 (2013). https://doi.org/10.1007/s11418-012-0685-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-012-0685-4