Abstract
In brains from patients with Alzheimer’s disease (AD), expression of insulin receptor (IR), insulin-like growth factor-1 receptor (IGF-1R), and insulin receptor substrate proteins is downregulated. A key step in the pathogenesis of AD is the accumulation of amyloid precursor protein (APP) cleavage products, β-amyloid (Aβ)1-42 and Aβ1–40. Recently, we and others have shown that central IGF-1 resistance reduces Aβ accumulation as well as Aβ toxicity and promotes survival. To define the role of IR in this context, we crossed neuron-specific IR knockout mice (nIR−/−) with Tg2576 mice, a well-established mouse model of an AD-like pathology. Here, we show that neuronal IR deficiency in Tg2576 (nIR−/−Tg2576) mice leads to markedly decreased Aβ burden but does not rescue premature mortality of Tg2576 mice. Analyzing APP C-terminal fragments (CTF) revealed decreased α-/β-CTFs in the brains of nIR−/−Tg2576 mice suggesting decreased APP processing. Cell based experiments showed that inhibition of the PI3-kinase pathway suppresses endosomal APP cleavage and decreases α- as well as β-secretase activity. Deletion of only one copy of the neuronal IGF-1R partially rescues the premature mortality of Tg2576 mice without altering total amyloid load. Analysis of Tg2576 mice expressing either a dominant negative or constitutively active form of forkhead box-O (FoxO)1 did not reveal any alteration of amyloid burden, APP processing and did not rescue premature mortality in these mice. Thus, our findings identified IR signaling as a potent regulator of Aβ accumulation in vivo. But exclusively decreased IGF-1R expression reduces AD-associated mortality independent of β-amyloid accumulation and FoxO1-mediated transcription.







Similar content being viewed by others
References
Adachi M, Osawa Y, Uchinami H, Kitamura T, Accili D, Brenner DA (2007) The forkhead transcription factor FoxO1 regulates proliferation and transdifferentiation of hepatic stellate cells. Gastroenterology 132:1434–1446
Adlerz L, Holback S, Multhaup G, Iverfeldt K (2007) IGF-1-induced processing of the amyloid precursor protein family is mediated by different signaling pathways. J Biol Chem 282:10203–10209
Baird PA, Sadovnick AD (1988) Life expectancy in Down syndrome adults. Lancet 2:1354–1356
Belgardt BF, Husch A, Rother E, Ernst MB, Wunderlich FT, Hampel B, Klockener T, Alessi D, Kloppenburg P, Bruning JC (2008) PDK1 deficiency in POMC-expressing cells reveals FoxO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab 7:291–301
Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR (2000) Role of brain insulin receptor in control of body weight and reproduction. Science 289:2122–2125
Burgering BM (2008) A brief introduction to FOXOlogy. Oncogene 27:2258–2262
Cabrejo L, Guyant-Marechal L, Laquerriere A, Vercelletto M, De la FF, Thomas-Anterion C, Verny C, Letournel F, Pasquier F, Vital A, Checler F, Frebourg T, Campion D, Hannequin D (2006) Phenotype associated with APP duplication in five families. Brain 129:2966–2976
Carlson GA, Borchelt DR, Dake A, Turner S, Danielson V, Coffin JD, Eckman C, Meiners J, Nilsen SP, Younkin SG, Hsiao KK (1997) Genetic modification of the phenotypes produced by amyloid precursor protein overexpression in transgenic mice. Hum Mol Genet 6:1951–1959
Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, George-Hyslop PS, Westaway D (2001) Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem 276:21562–21570
Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A (2006) Opposing activities protect against age-onset proteotoxicity. Science 313:1604–1610
Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW, Masliah E, Dillin A (2009) Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell 139:1157–1169
Cohen E, Du D, Joyce D, Kapernick EA, Volovik Y, Kelly JW, Dillin A (2010) Temporal requirements of insulin/IGF-1 signaling for proteotoxicity protection. Aging Cell 9:126–134
Costantini C, Scrable H, Puglielli L (2006) An aging pathway controls the TrkA to p75NTR receptor switch and amyloid beta-peptide generation. EMBO J 25:1997–2006
Craft S (2009) The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol 66:300–305
Crews L, Masliah E (2010) Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum Mol Genet 19:R12–R20
Day SM, Strauss DJ, Shavelle RM, Reynolds RJ (2005) Mortality and causes of death in persons with Down syndrome in California. Dev Med Child Neurol 47:171–176
de la Monte SM, Tong M, Lester-Coll N, Plater M Jr, Wands JR (2006) Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer’s disease. J Alzheimers Dis 10:89–109
Douglas PM, Dillin A (2010) Protein homeostasis and aging in neurodegeneration. J Cell Biol 190:719–729
El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD (2007) Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med 13:432–438
Finch CE, Ruvkun G (2001) The genetics of aging. Annu Rev Genomics Hum Genet 2:435–462
Freude S, Hettich MM, Schumann C, Stohr O, Koch L, Kohler C, Udelhoven M, Leeser U, Muller M, Kubota N, Kadowaki T, Krone W, Schroder H, Bruning JC, Schubert M (2009) Neuronal IGF-1 resistance reduces A{beta} accumulation and protects against premature death in a model of Alzheimer’s disease. FASEB J 23:3315–3324
Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Turk A, Hoyer S, Zochling R, Boissl KW, Jellinger K, Riederer P (1998) Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm 105:423–438
Frolich L, Blum-Degen D, Riederer P, Hoyer S (1999) A disturbance in the neuronal insulin receptor signal transduction in sporadic Alzheimer’s disease. Ann N Y Acad Sci 893:290–293
Furuyama T, Nakazawa T, Nakano I, Mori N (2000) Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J 349:629–634
Greeve I, Kretzschmar D, Tschape JA, Beyn A, Brellinger C, Schweizer M, Nitsch RM, Reifegerste R (2004) Age-dependent neurodegeneration and Alzheimer-amyloid plaque formation in transgenic Drosophila. J Neurosci 24:3899–3906
Hermon C, Alberman E, Beral V, Swerdlow AJ (2001) Mortality and cancer incidence in persons with Down’s syndrome, their parents and siblings. Ann Hum Genet 65:167–176
Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A, Mobbs CV, Hof PR, Pasinetti GM (2004) Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. FASEB J 18:902–904
Hoekman MF, Jacobs FM, Smidt MP, Burbach JP (2006) Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr Patterns 6:134–140
Hsiao KK, Borchelt DR, Olson K, Johannsdottir R, Kitt C, Yunis W, Xu S, Eckman C, Younkin S, Price D (1995) Age-related CNS disorder and early death in transgenic FVB/N mice overexpressing Alzheimer amyloid precursor proteins. Neuron 15:1203–1218
Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G (1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274:99–102
Isacson O, Seo H, Lin L, Albeck D, Granholm AC (2002) Alzheimer’s disease and Down’s syndrome: roles of APP, trophic factors and ACh. Trends Neurosci 25:79–84
Jacobs FM, van der Heide LP, Wijchers PJ, Burbach JP, Hoekman MF, Smidt MP (2003) FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J Biol Chem 278:35959–35967
Jacobsen KT, Adlerz L, Multhaup G, Iverfeldt K (2010) Insulin-like growth factor-1 (IGF-1)-induced processing of amyloid-beta precursor protein (APP) and APP-like protein 2 is mediated by different metalloproteinases. J Biol Chem 285:10223–10231
Kappeler L, Filho CM, Dupont J, Leneuve P, Cervera P, Perin L, Loudes C, Blaise A, Klein R, Epelbaum J, Le Bouc Y, Holzenberger M (2008) Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biol 6:e254-
Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R (1993) A C. elegans mutant that lives twice as long as wild type. Nature 366:461–464
Killick R, Scales G, Leroy K, Causevic M, Hooper C, Irvine EE, Choudhury AI, Drinkwater L, Kerr F, Al Qassab H, Stephenson J, Yilmaz Z, Giese KP, Brion JP, Withers DJ, Lovestone S (2009) Deletion of Irs2 reduces amyloid deposition and rescues behavioural deficits in APP transgenic mice. Biochem Biophys Res Commun 386:257–262
Kitamura T, Kitamura YI, Funahashi Y, Shawber CJ, Castrillon DH, Kollipara R, DePinho RA, Kitajewski J, Accili D (2007) A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J Clin Invest 117:2477–2485
Kroner Z (2009) The relationship between Alzheimer’s disease and diabetes: type 3 diabetes? Altern Med Rev 14:373–379
Lee RY, Hench J, Ruvkun G (2001) Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol 11:1950–1957
Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ (2003) Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40:1087–1093
Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH (2006) A specific amyloid-beta protein assembly in the brain impairs memory. Nature 440:352–357
Lin K, Dorman JB, Rodan A, Kenyon C (1997) daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278:1319–1322
Lovestone S (1999) Diabetes and dementia: is the brain another site of end-organ damage? Neurology 53:1907–1909
Marks N, Berg MJ (2010) BACE and gamma-secretase characterization and their sorting as therapeutic targets to reduce amyloidogenesis. Neurochem Res 35:181–210
Matsubara E, Bryant-Thomas T, Pacheco QJ, Henry TL, Poeggeler B, Herbert D, Cruz-Sanchez F, Chyan YJ, Smith MA, Perry G, Shoji M, Abe K, Leone A, Grundke-Ikbal I, Wilson GL, Ghiso J, Williams C, Refolo LM, Pappolla MA, Chain DG, Neria E (2003) Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer’s disease. J Neurochem 85:1101–1108
Meilandt WJ, Cisse M, Ho K, Wu T, Esposito LA, Scearce-Levie K, Cheng IH, Yu GQ, Mucke L (2009) Neprilysin overexpression inhibits plaque formation but fails to reduce pathogenic A{beta} oligomers and associated cognitive deficits in human amyloid precursor protein transgenic mice. J Neurosci 29:1977–1986
Moechars D, Dewachter I, Lorent K, Reverse D, Baekelandt V, Naidu A, Tesseur I, Spittaels K, Haute CV, Checler F, Godaux E, Cordell B, Van Leuven F (1999) Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem 274:6483–6492
Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C (2008) Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging 31(2):224–243
Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C (2010) Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging 31:224–243
Nathan C, Calingasan N, Nezezon J, Ding A, Lucia MS, La Perle K, Fuortes M, Lin M, Ehrt S, Kwon NS, Chen J, Vodovotz Y, Kipiani K, Beal MF (2005) Protection from Alzheimer’s-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J Exp Med 202:1163–1169
Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G (1997) The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389:994–999
Ott A, Stolk RP, Hofman A, van Harskamp F, Grobbee DE, Breteler MM (1996) Association of diabetes mellitus and dementia: the Rotterdam Study. Diabetologia 39:1392–1397
Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM (1999) Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology 53:1937–1942
Pilcher H (2006) Alzheimer’s disease could be “type 3 diabetes”. Lancet Neurol 5:388–389
Puglielli L (2008) Aging of the brain, neurotrophin signaling, and Alzheimer’s disease: is IGF1-R the common culprit? Neurobiol Aging 29:795–811
Puglielli L, Ellis BC, Saunders AJ, Kovacs DM (2003) Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. J Biol Chem 278:19777–19783
Rajendran L, Schneider A, Schlechtingen G, Weidlich S, Ries J, Braxmeier T, Schwille P, Schulz JB, Schroeder C, Simons M, Jennings G, Knolker HJ, Simons K (2008) Efficient inhibition of the Alzheimer’s disease beta-secretase by membrane targeting. Science 320:520–523
Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM (2005) Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis 8:247–268
Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerriere A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, Dubas F, Frebourg T, Campion D (2006) APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet 38:24–26
Sleegers K, Brouwers N, Gijselinck I, Theuns J, Goossens D, Wauters J, Del Favero J, Cruts M, van Duijn CM, Van Broeckhoven C (2006) APP duplication is sufficient to cause early onset Alzheimer’s dementia with cerebral amyloid angiopathy. Brain 129:2977–2983
Stachelscheid H, Ibrahim H, Koch L, Schmitz A, Tscharntke M, Wunderlich FT, Scott J, Michels C, Wickenhauser C, Haase I, Bruning JC, Niessen CM (2008) Epidermal insulin/IGF-1 signalling control interfollicular morphogenesis and proliferative potential through Rac activation. EMBO J 27:2091–2101
Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM (2005) Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes? J Alzheimers Dis 7:63–80
Stein TD, Johnson JA (2002) Lack of neurodegeneration in transgenic mice overexpressing mutant amyloid precursor protein is associated with increased levels of transthyretin and the activation of cell survival pathways. J Neurosci 22:7380–7388
Strauss D, Eyman RK (1996) Mortality of people with mental retardation in California with and without Down syndrome, 1986–1991. Am J Ment Retard 100:643–653
Taguchi A, Wartschow LM, White MF (2007) Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 317:369–372
Willuweit A, Velden J, Godemann R, Manook A, Jetzek F, Tintrup H, Kauselmann G, Zevnik B, Henriksen G, Drzezga A, Pohlner J, Schoor M, Kemp JA, von der Kammer K (2009) Early-onset and robust amyloid pathology in a new homozygous mouse model of Alzheimer’s disease. PLoS One 4:e7931
Wisniewski KE, Wisniewski HM, Wen GY (1985) Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol 17:278–282
Wolkow CA, Kimura KD, Lee MS, Ruvkun G (2000) Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science 290:147–150
Yang Q, Rasmussen SA, Friedman JM (2002) Mortality associated with Down’s syndrome in the USA from 1983 to 1997: a population-based study. Lancet 359:1019–1025
Acknowledgments
We thank Prof. Dr. R. Wiesner for critical discussion of the data and the manuscript. This work was supported by the Alzheimer Forschungsinitiative e.V. (to MS # 08813) and the Else-Kröner-Fresenius Stiftung (to MS #2010_A93) and Köln Fortune (to MS and KS)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Oliver Stöhr, Katharina Schilbach, and Lorna Moll contributed equally to this work.
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
a Mutant FoxO1 constructs. b Cloning strategy of FoxO1DN and FoxO1ADA mice, IRES-internal ribosomal entry site, and WSS westphal stop sequence. c Immunohistochemistry using antibodies against eGFP in Rosa FoxO1DN Syn-Cre mice and respective controls (JPEG 88 kb)
Supplementary Fig. 2
Insulin-stimulated Akt and Erk-1/-2 phosphorylation is decreased in hippocampi from nIR−/− mice. Isolated hippocampi of WT and nIR−/− mice were stimulated with insulin (5 nM) for 10 min. Hippocampal lysates were subject to SDS-PAGE and Western blotting. Membranes were probed using antbodies against pAktser473, Akt, pErk-1/-2Thr202/Tyr204, and Erk-1/-2 (JPEG 13 kb)
Supplementary Fig. 3
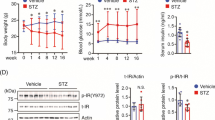
Characterization of nIR−/−Tg2576 mice and respective controls up to the age of 60 weeks. a Body weight from male and female animals. b Blood glucose levels from male and female animals. Data were assessed from 66 female (50 Tg2576 and 16 nIR−/−Tg2576) and 66 male mice (52 Tg2576 and 14 nIR−/−Tg2576) (JPEG 44 kb)
Supplementary Fig. 4
Reduced IGF-1R expression and decreased IGF-1-stimulated Akt phosphorylation in isolated hippocampi from nIGF-R+/− mice. a Determination of the abundance of IGF-1Rs from hippocampal lysates of WT and nIGF-R+/− mice using Western blots. b Isolated hippocampi of WT and nIGF-R+/− mice were stimulated with IGF-1 (5 nM) for 10 min. Hippocampal lysates were subject to SDS-PAGE and Western blotting. Membranes were probed using antibodies against pAktser473 and Akt (JPEG 15 kb)
Supplementary Fig. 5
Characterization of nIGF-R+/−Tg2576 mice and respective controls up to the age of 60 weeks. a Body weight from male and female animals. b Blood glucose levels from male and female animals. Data were assessed from 50 female (40 Tg2576 and 10 nIR−/−Tg2576) and 49 male mice (31 Tg2576 and 18 nIR−/−Tg2576) (JPEG 41 kb)
Supplementary Fig. 6
Characterization of FoxO1ADATg2576 mice and respective controls up to the age of 60 weeks. a Body weight from male and female animals. b Blood glucose levels from male and female animals. Data were assessed from 50 female (37 Tg2576 and 13 FoxO1ADATg2576) and 39 male mice (37 Tg2576 and 2 FoxO1ADATg2576). c Western blots of hippocampal lysates for the IGF-1R, pAktser473, Akt, pErk-1/-2Thr202/Tyr204, and Erk-1/-2 from female WT, FoxO1ADATg2576, FoxO1ADA, and Tg2576 mice (n = 4 per genotype) (JPEG 36 kb)
Supplementary Fig. 7
Characterization of FoxO1DNTg2576 mice and respective controls up to the age of 60 weeks. a Body weight from male and female animals. b Blood glucose levels from male and female animals. Data were assessed from 70 female (56 Tg2576 and 14 FoxO1DNTg2576) and 56 male mice (44 Tg2576 and 12 FoxO1DNTg2576). c Western blot analysis for IGF-1R, IR, pAktser473, Akt, pErk-1/-2Thr202/Tyr204, Erk-1/-2, and FoxO1 from hippocampal lysates of WT, FoxO1DNTg2576, FoxO1DN, and Tg2576 mice (n = 4 per genotype) (JPEG 38 kb)
About this article
Cite this article
Stöhr, O., Schilbach, K., Moll, L. et al. Insulin receptor signaling mediates APP processing and β-amyloid accumulation without altering survival in a transgenic mouse model of Alzheimer’s disease. AGE 35, 83–101 (2013). https://doi.org/10.1007/s11357-011-9333-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-011-9333-2