Abstract
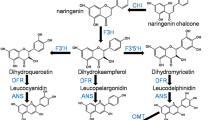
Detection and quantification of the levels of adventitious presence of genetically modified (GM) soybeans in non-GM grain shipments currently requires sophisticated tests that can have issues with their reproducibility. We show here that pigment biosynthesis in the soybean seed coat can be manipulated to provide a distinct color that would enable the simple visible detection of the GM soybean grain. We observed that a distinct red-brown grain color could be engineered by the simultaneous suppression of two proanthocyanidin (PA) genes, ANTHOCYANIDIN REDUCTASE1 (ANR1) and ANR2. Multiple reaction monitoring by liquid chromatography tandem mass spectrometry was used to quantify differentially accumulated seed coat metabolites, and revealed the redirection of metabolic flux into the anthocyanin pigment pathway and unexpectedly the flavonol-3-O-glucoside pathway. The upregulations of anthocyanin isogenes (DFR1 and GST26) and the anthocyanin/flavonol-3-O-glycosyltransferase (UGT78K2) were identified by quantitative RT-PCR to be endogenous feedback and feedforward responses to overaccumulation of upstream flavonoid intermediates resulting from ANR1 and ANR2 suppressions. These results suggested the transcription of flavonoid genes to be a key component of the mechanism responsible for the redirection of metabolite flux. This report identifies the suppression of PA genes to be a novel approach for engineering pigmentation in soybean grains.






Similar content being viewed by others
References
Albert S, Delseny M, Devic M (1997) BANYULS, a novel negative regulator of flavonoid biosynthesis in the Arabidopsis seed coat. Plant J 11:289–299
Basaran P, Rodriguez-Cerezo E (2008) Plant molecular farming: opportunities and challenges. Crit Rev Biotechnol 28:153–172
Besseau S, Hoffmann L, Geoffroy P, Lapierre C, Pollet B, Legrand M (2007) Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell 19:148–162
Blount JW, Korth KL, Masoud SA, Rasmussen S, Lamb C, Dixon RA (2000) Altering expression of cinnamic acid 4-hydroxylase in transgenic plants provides evidence for a feedback loop at the entry point into the phenylpropanoid pathway. Plant Physiol 122:107–116
Bolwell G, Cramer C, Lamb C, Schuch W, Dixon R (1986) l-Phenylalanine ammonia-lyase from Phaseolus vulgaris: modulation of the levels of active enzyme by trans-cinnamic acid. Planta 149:97–107
Demeke T, Perry D, Scowcroft W (2006) Adventitious presence of GMOs: scientific overview for Canadian grains. Can J Plant Sci 86:1–23
Devic M, Guilleminot J, Debeaujon I, Bechtold N, Bensaude E, Koornneef M, Pelletier G, Delseny M (1999) The BANYULS gene encodes a DFR-like protein and is a marker of early seed coat development. Plant J 19:387–398
Finer JJ, McMullen MD (1991) Transformation of soybean via particle bombardment of embryogenic suspension culture tissue. In Vitro Cell Dev Biol Plant 27:175–182
Geekiyanage S (2009) Potential of VlmybAl-2 as a candidate marker for visual identification of transgenic plants: induced anthocyanin production in Arabidopsis and tobacco. Trop Agric Res Ext 12:35–41
Hahlbrock K, Knobloch K, Kreuzaler F, Potts J, Wellmann E (1976) Coordinated induction and subsequent activity changes of two groups of metabolically interrelated enzymes. Light-induced synthesis of flavonoid glycosides in cell suspension cultures of Petroselinum hortense. Eur J Biochem 61:199–206
Hartmann U, Sagasser M, Mehrtens F, Stracke R, Weisshaar B (2005) Differential combinatorial interactions of cis-acting elements recognized by R2R3-MYB, BZIP, and BHLH factors control light-responsive and tissue-specific activation of phenylpropanoid biosynthesis genes. Plant Mol Biol 57:155–171
Holton TA (1995) Modification of flower colour via manipulation of P450 gene expression in transgenic plants. Drug Metabol Drug Interact 12:359–368
Holton TA, Brugliera F, Tanaka Y (1993) Cloning and expression of flavonol synthase from Petunia hybrida. Plant J 4:1003–1010
Karg SR, Kallio PT (2009) The production of biopharmaceuticals in plant systems. Biotechnol Adv 27:879–894
Kovinich N, Saleem A, Arnason JT, Miki B (2010) Functional characterization of a UDP-glucose:flavonoid 3-O-glucosyltransferase from the seed coat of black soybean (Glycine max (L.) Merr.). Phytochemistry 71:1253–1263
Kovinich N, Arnason JT, De Luca V, Miki B (2011a) Coloring soybeans with anthocyanins? In: Gang DR (ed) Recent advances in phytochemistry. Springer, New York, pp 47–57
Kovinich N, Saleem A, Arnason JT, Miki B (2011b) Combined analysis of transcriptome and metabolite data reveals extensive differences between black and brown nearly-isogenic soybean (Glycine max) seed coats enabling the identification of pigment isogenes. BMC Genomics 12:381
Kovinich N, Saleem A, Arnason JT, Miki B (Submitted) Identification of two anthocyanidin reductase genes from the seed coat of brown soybean and three red-brown soybean accessions that have reduced ANTHOCYANIDIN REDUCTASE1 mRNA, activity, and seed coat proanthocyanidin amounts. J Agric Food Chem
Krueger R, Le Buanec B (2008) Action needed to harmonize regulation of low-level presence of biotech traits. Nat Biotechnol 26:161–162
Lee Y, Yoon H, Paik YS, Liu JR, Chung W-I, Choi G (2005) Reciprocal regulation of Arabidopsis UGT78D2 and BANYULS is critical for regulation of the metabolic flux of anthocyanidins to condensed tannins in developing seed coats. J Plant Biol 48:356–370
Lemaux PG (2008) Genetically engineered plants and foods: a scientist’s analysis of the issues (part I). Annu Rev Plant Biol 59:771–812
Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M (2006) Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol 57:405–430
Loake GJ, Choudhary AD, Harrison MJ, Mavandad M, Lamb CJ, Dixon RA (1991) Phenylpropanoid pathway intermediates regulate transient expression of a chalcone synthase gene promoter. Plant Cell 3:829–840
McCallum JA, Walker JRL (1990) Proanthocyanidins in wheat bran. Cereal Chem 67:282–285
Meyer P, Heidmann I, Forkmann G, Saedler H (1987) A new petunia flower colour generated by transformation of a mutant with a maize gene. Nature 330:677–678
Miki D, Shimamoto K (2004) Simple RNAi vectors for stable and transient suppression of gene function in rice. Plant Cell Physiol 45:490–495
Mol J, Grotewold E, Koesa R (1998) How genes paint flowers and seeds. Trends Plant Sci 3:212–217
Morisset D, Demsar T, Gruden K, Vojvoda J, Stebih D, Zel J (2009) Detection of genetically modified organisms-closing the gaps. Nat Biotechnol 27:700–701
Naczk M, Nichols T, Pink D, Sosulski F (1994) Condensed tannins in Canola Hulls. J Agric Food Chem 42:2196–2200
Nagamatsu A, Masuta C, Matsuura H, Kitamura K, Abe J, Kanazawa A (2009) Down-regulation of flavonoid 3′-hydroxylase gene expression by virus-induced gene silencing in soybean reveals the presence of a threshold mRNA level associated with pigmentation in pubescence. J Plant Physiol 166:32–39
Nakatsuka T, Abe Y, Kakizaki Y, Yamamura S, Nishihara M (2007) Production of red-flowered plants by genetic engineering of multiple flavonoid biosynthetic genes. Plant Cell Rep 26:1951–1959
Nishihara M, Nakatsuka T (2011) Genetic engineering of flavonoid pigments to modify flower color in floricultural plants. Biotechnol Lett 33:433–441
Pelletier MK, Murrell JR, Shirley BW (1997) Characterization of flavonol synthase and leucoanthocyanidin dioxygenase genes in Arabidopsis. Further evidence for differential regulation of “early” and “late” genes. Plant Physiol 113:1437–1445
Ramessar K, Capell T, Twyman RM, Christou P (2010) Going to ridiculous lengths—European coexistence regulations for GM crops. Nat Biotechnol 28:133–136
Routaboul JM, Kerhoas L, Debeaujon I, Pourcel L, Caboche M, Einhorn J, Lepiniec L (2006) Flavonoid diversity and biosynthesis in seed of Arabidopsis thaliana. Planta 224:96–107
Shen L, Petolino J (2006) Pigmented maize seed via tissue-specific expression of anthocyanin pathway gene transcription factors. Mol Breed 18:57–67
Smyth S, McHughen A (2008) Regulating innovative crop technologies in Canada: the case of regulating genetically modified crops. Plant Biotechnol J 6:213–225
Stewart CN Jr (2005) Monitoring the presence and expression of transgenes in living plants. Trends Plant Sci 10:390–396
Stewart CN Jr (2006) Go with the glow: fluorescent proteins to light transgenic organisms. Trends Biotechnol 24:155–162
Stracke R, Ishihara H, Huep G, Barsch A, Mehrtens F, Niehaus K, Weisshaar B (2007) Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J 50:660–677
Stracke R, De Vos RC, Bartelniewoehner L, Ishihara H, Sagasser M, Martens S, Weisshaar B (2009) Metabolomic and genetic analyses of flavonol synthesis in Arabidopsis thaliana support the in vivo involvement of leucoanthocyanidin dioxygenase. Planta 229:427–445
Sugimoto T, Kawasaki T, Kato T, Whittier RF, Shibata D, Kawamura Y (1992) cDNA sequence and expression of a phosphoenolpyruvate carboxylase gene from soybean. Plant Mol Biol 20:743–747
Tanaka Y, Tsuda S, Kusumi T (1998) Metabolic engineering to modify flower color. Plant Cell Physiol 39:1119–1126
Tsuda S, Fukui Y, Nakamura N, Katsumoto Y, Yonekura-Sakakibara K, Fukuchi-Mizutani M, Ohira K, Ueyama Y, Ohkawa H, Holton TA, Kusumi T, Tanaka Y (2004) Flower color modification of Petunia hybrida commercial varieties by metabolic engineering. Plant Biotechnol 21:377–386
Tuteja JH, Clough SJ, Chan WC, Vodkin LO (2004) Tissue-specific gene silencing mediated by a naturally occurring chalcone synthase gene cluster in Glycine max. Plant Cell 16:819–835
Venglat P, Xiang D, Qiu S, Stone SL, Tibiche S, Cram D, Alting-Mees M, Nowak J, Cloutier S, Deyholos M, Bekkaoui F, Sharpe A, Wang E, Rowland G, Selvaraj G, Datla R (2011) Gene expression analysis of flax seed development. BMC Plant Biol 11:74
Wang C-S, Vodkin LO (1994) Extraction of RNA from tissues containing high levels of procyanidins that bind RNA. Plant Mol Biol Rep 12:132–145
Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493
Xie DY, Sharma SB, Paiva NL, Ferreira D, Dixon RA (2003) Role of anthocyanidin reductase, encoded by BANYULS in plant flavonoid biosynthesis. Science 299:396–399
Yu O, Shi J, Hession AO, Maxwell CA, McGonigle B, Odell JT (2003) Metabolic engineering to increase isoflavone biosynthesis in soybean seed. Phytochemistry 63:753–763
Zhao J, Dixon RA (2010) The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci 15:72–80
Zhao J, Huhman D, Shadle G, He XZ, Sumner LW, Tang Y, Dixon RA (2011) MATE2 mediates vacuolar sequestration of flavonoid glycosides and Glycoside Malonates in Medicago truncatula. Plant Cell 23:1536–1555
Acknowledgments
We gratefully thank Dr. Vincenzo De Luca for the fruitful conversations, Dr. Ko Shimamoto for providing the pANDA35HK vector and Carla Schmidt for assistance with soybean transformation. This project was funded by the A-base (project inventory 126) from Agriculture and Agri-Food Canada to BM and DB, and NSERC Discovery Grants to BM and JTA.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kovinich, N., Saleem, A., Rintoul, T.L. et al. Coloring genetically modified soybean grains with anthocyanins by suppression of the proanthocyanidin genes ANR1 and ANR2 . Transgenic Res 21, 757–771 (2012). https://doi.org/10.1007/s11248-011-9566-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-011-9566-y