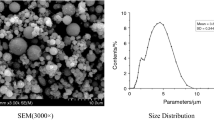
Results are given for development of crystalline α-Al2O3 phase, crystal dimensions, degree of corundum phase crystallization microstructure, powder specific surface, and particle size distribution, obtained in the course of combustion of components in dry mixes using aluminum powder and different oxidizing agents. Aluminum powder combustion in the presence of oxidizing agents in the field from potassium nitrate to potassium perchlorate facilitates formation of broader, crystalline, and simultaneously intense diffraction maxima for α-Al2O3 phase. This is reflected in smaller crystal sizes and degree of corundum phase crystallization. Development of powder microstructure is governed by densely sintered particles, consisting of soft and hard agglomerates. It is relatively porous in the course of combustion with participation of strong oxidizing agents. This shows up as a less developed specific powder surface and wider particle size distribution, located in a field of coarse fractions in the range 5 – 54 μm, in contrast to powder prepared using potassium nitrate, whose particle sizes are located in the range 0.5 – 1.2 μm.




Similar content being viewed by others
References
V. S. Gorshkov and V. G. Solov’ev, Physical Chemistry of Silicates and Other Refractory Compounds [in Russian], Vysshaya Shkola, Kiev (1988).
A. G. Merzhanov, “History and recent developments in SHS,” Ceram. Internat., 21(5), 371 – 379 (1995).
M. Halmann, A. Frei, and A. Steinfeld, “Carbothermal reduction of alumina: Thermochemical equilibrium calculations and experimental investigation,” Energy, 32(12), 2420 – 2427 (2007).
S. T. Aruna and A. S. Mukasyan Aruna, “Combustion synthesis and nanomaterials,” Current Opinion in Solid State and Materials Science, 12(3/4), 44 – 50 (2008).
A. A. Pashchenko, Physical Chemistry of Silicates [in Russian], Vysshaya Shkola, Kiev (1986).
C. L. Yeh and H. J. Wang, “Combustion synthesis of vanadium borides,” J. All. Comp., 509(7), 3257 – 3261 (2011).
C. L. Yeh, F. S.Wi, and Y. L. Chen “Effects of α- and β-Si3N4 as precursors on combustion synthesis of (α+β)-SiAlON composites,” J. All. Comp., 509(9), 3985 – 3990 (2011).
L. Durach, R. Santos, and A. Correia, “Fe2O3 / aluminium thermite reaction intermediate and final products characterization,” J. Comp. Mat. Sci. Eng. A., 465(1/2), 199 – 210 (2007).
H. Hassan, S. A. Mehdi, and E. Mehri, “Synthesis of titanium carbide by the combustion of TiO2–2 Mg–C and 3TiO2–4Al–3C systems in a tubular furnace,” Iran J. Chem. Eng., 28(1), 71 – 76 (2009).
K. C. Patil, S. T. Aruna, and T. Mimani, “Combustion synthesis: an update,” Current Opinion in Solid State and Materials Science, 12(6), 507 – 512 (2002).
S. M. Pourmartazavi, S. S. Hajimirsadeghi, and S. G. Hossein, “Characterization of the aluminium / potassium chlorate mixtures by simultaneous TG-DTA,” J. Thermal Analysis and Calorimetry, 84(3), 557 – 561 (2006).
A. N. Pivkina, Yu. V. Frolov, and D. A. Ivanov, “Nanosized components of energetic systems: structure, thermal behaviour, and combustion,” Combustion, Explosion, and Shock Waves, 43(1), 51 – 55 (2007).
V. Arkhipov, M. Gorbenko, T. Gorbenko, et al., “Effect of ultrafine aluminium on the combustion of composite solid propellants at subatmospheric pressures,” Combustion, Explosion, and Shock Waves, 45(1), 40 – 47 (2009).
E. L. Preizan, “Metal-based reactive nanomaterials,” Progress in Energy and Combustion Science, 35(2), 141 – 167 (2009).
V. D. Zhuravlev, V. G. Bamburov, A. R. Beketov, et al., “Solution combustion synthesis of αAl2O3 using urea,” Ceram. Internat., 39(2), 1379 – 1384 (2013).
Author information
Authors and Affiliations
Additional information
Translated from Novye Ogneupory, No. 12, pp. 25 – 29, December, 2013.
Rights and permissions
About this article
Cite this article
Khmelev, A.V. Use of SHS in Metal and Oxidizing Agent Dry Powder Mixes. Refract Ind Ceram 54, 485–489 (2014). https://doi.org/10.1007/s11148-014-9638-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-014-9638-7