Abstract
Objective
The goal of this paper is to discuss cancer-related fatigue (CRF) and address issues related to the investigation into potential biological and genetic causal mechanisms. The objectives are to: (1) describe CRF as a component of quality of life (QOL); (2) address measurement issues that have slowed progress toward an understanding of mechanisms underlying this symptom; (3) review biological pathways and genetic approaches that have promise for the exploration of causal mechanisms of CRF; and (4) offer directions for future research.
Methods
Review, synthesis, and interpretation of the literature.
Results
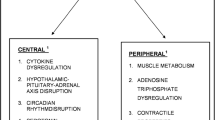
Until recently, CRF and QOL have been understood primarily as subjective patient-reported experiences. With increased understanding of human genetics, theories and research are being expanded to incorporate biological and genetic understandings of these subjective experiences. Proposed biological and genetic mechanisms of CRF that have been examined include cytokine dysregulation, hypothalamic–pituitary–adrenal (HPA) axis dysfunction, five hydroxy tryptophan (5-HT) neurotransmitter dysregulation, circadian rhythm disruption, alterations in adenosine triphosphate (ATP) and muscle metabolism, and vagal afferent activation. Approaches to the study of genetic mechanisms have also been addressed including candidate genes, genome-wide scanning, and gene expression. Based on the review and synthesis of the literature, directions for future research are proposed.
Conclusions
Understanding the biological and genetic basis of CRF has the potential to contribute to a more complete understanding of the genetic determinants of QOL.
Similar content being viewed by others
References
Higginson, I. J., Armes, J., & Krishnasamy, M. (2004). Introduction, in fatigue in cancer. In J. Armes, M. Krishnasamy, & I. J. Higginson (Eds.), Fatigue in cancer (pp. xvii–xxi). Oxford: Oxford University Press.
Cella, D., Davis, K., Breitbart, W., & Curt, G. (2001). Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. Journal of Clinical Oncology, 19(14), 3385–3391.
Servaes, P., Verhagen, C., & Bleijenberg, G. (2002). Fatigue in cancer patients during and after treatment: Prevalence, correlates and interventions. European Journal of Cancer, 38(1), 27–43.
Smets, E. M., Garssen, B., Cull, A., & de Haes, J. C. (1996). Application of the multidimensional fatigue inventory (MFI-20) in cancer patients receiving radiotherapy. British Journal of Cancer, 73(2), 241–245.
Curt, G. A., Breitbart, W., Cella, D., Groopman, J. E., Horning, S. J., Itri, L. M., et al. (2000). Impact of cancer-related fatigue on the lives of patients: New findings from the Fatigue Coalition. The Oncologist, 5(5), 353–360.
Lawrence, D. P., Kupelnick, B., Miller, K., Devine, D., & Lau, J. (2004). Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. Journal of the National Cancer Institute Monographs, 32, 40–50.
Vogelzang, N. J., Breitbart, W., Cella, D., Curt, G. A., Groopman, J. E., Horning, S. J., et al. (1997). Patient, caregiver, and oncologist perceptions of cancer-related fatigue: Results of a tripart assessment survey. The Fatigue Coalition. Seminars in Hematology, 34(3 Suppl 2), 4–12.
Wilson, I. B., & Cleary, P. D. (1995). Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA, 273(1), 59–65.
Sprangers, M. A. G., Sloan, J. A., Barsevick, A., Chauhan, C., Dueck, A. C., Raat H., et al. (2010). Scientific Imperatives, clinical implications, and theoretical underpinnings for the investigation of the relationship between genetic variables and patient-reported quality-of-life outcomes. Quality of Life Research. doi:10.1007/s11136-010-9759-5.
Andrykowski, M. A., Schmidt, J. E., Salsman, J. M., Beacham, A. O., & Jacobsen, P. B. (2005). Use of a case definition approach to identify cancer-related fatigue in women undergoing adjuvant therapy for breast cancer. Journal of Clinical Oncology, 23(27), 6613–6622.
Alexander, S., Minton, O., Andrews, P., & Stone, P. (2009). A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. European Journal of Cancer, 45(3), 384–392.
NCCN. (2010). The complete library of NCCN clinical practice guidelines in oncology. Fort Washington, PA: National Comprehensive Cancer Network.
Barsevick, A. M., Cleeland, C. S., Manning, D. C., O’Mara, A. M., Reeve, B. B., Scott, J. A., et al. (2010). ASCPRO Recommendations for the assessment of fatigue as an outcome in clinical trials. Journal of Pain and Symptom Management, 39(6), 1086–1099.
Mendoza, T. R., Wang, X. S., Cleeland, C. S., Morrissey, M., Johnson, B. A., Wendt, J. K., et al. (1999). The rapid assessment of fatigue severity in cancer patients: Use of the brief fatigue inventory. Cancer, 85(5), 1186–1196.
Piper, B. F., Lindsey, A. M., & Dodd, M. J. (1989). The development of an instrument to measure the subjective dimension of fatigue. In E. M. T. S. G. Funk & M. T. Campagne (Eds.), Key aspects of comfort: Management of pain, fatigue, and nausea (pp. 199–208). New York: Springer.
Schwartz, A. L. (1998). The schwartz cancer fatigue scale: Testing reliability and validity. Oncology Nursing Forum, 25(4), 711.
Smets, E. M., Garssen, B., Bonke, B., & De Haes, J. C. (1995). The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. Journal of Psychosomatic Research, 39(3), 315–325.
Stein, K. D., Martin, S. C., Hann, D. M., & Jacobsen, P. B. (1998). A multidimensional measure of fatigue for use with cancer patients. Cancer Practice, 6(3), 143–152.
Belza, B. L. (1995). Comparison of self-reported fatigue in rheumatoid arthritis and controls. Journal of Rheumatology, 22(4), 639–643.
Okuyama, T., Akechi, T., Kugaya, A., Okamura, H., Shima, Y., Maruguchi, M., et al. (2000). Development and validation of the cancer fatigue scale: A brief, three-dimensional, self-rating scale for assessment of fatigue in cancer patients. Journal of Pain and Symptom Management, 19(1), 5–14.
Lee, K. A., Hicks, G., & Nino-Murcia, G. (1991). Validity and reliability of a scale to assess fatigue. Psychiatry Research, 36(3), 291–298.
Ware, J. E., Jr., Snow, K. K., Kosinski, M., & Gandek, B. (1993). SF-36 health survey manual and interpretation guide. Boston: The Health Institute, New England Medical Center.
Alexander, S., Minton, O., Stone, P. C., Alexander, S., Minton, O., & Stone, P. C. (2009). Evaluation of screening instruments for cancer-related fatigue syndrome in breast cancer survivors. Journal of Clinical Oncology, 27(8), 1197–1201.
Sadler, I. J., Jacobsen, P. B., Booth-Jones, M., Belanger, H., Weitzner, M. A., & Fields, K. K. (2002). Preliminary evaluation of a clinical syndrome approach to assessing cancer-related fatigue. Journal of Pain and Symptom Management, 23(5), 406–416.
Gutstein, H. B. (2001). The biologic basis of fatigue. Cancer, 92(6 Suppl), 1678–1683.
Ryan, J. L., Carroll, J. K., Ryan, E. P., Mustian, K. M., Fiscella, K., & Morrow, G. R. (2007). Mechanisms of cancer-related fatigue. The Oncologist, 12(Suppl 1), 22–34.
Morrow, G. R., Andrews, P. L., Hickok, J. T., Roscoe, J. A., Matteson, S., Morrow, G. R., et al. (2002). Fatigue associated with cancer and its treatment. Supportive Care in Cancer, 10(5), 389–398.
Bower, J. E., Ganz, P. A., Aziz, N., Fahey, J. L., Cole, S. W., Bower, J. E., et al. (2003). T-cell homeostasis in breast cancer survivors with persistent fatigue. Journal of the National Cancer Institute, 95(15), 1165–1168.
Greenberg, D. B., Gray, J. L., Mannix, C. M., Eisenthal, S., & Carey, M. (1993). Treatment-related fatigue and serum interleukin-1 levels in patients during external beam irradiation for prostate cancer. Journal of Pain and Symptom Management, 8(4), 196–200.
Bower, J. E., Ganz, P. A., Aziz, N., & Fahey, J. L. (2002). Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosomatic Medicine, 64(4), 604–611.
Levy, S., Herberman, R., Lippman, M., & d’Angelo, T. (1987). Correlation of stress factors with sustained depression of natural killer cell activity and predicted prognosis in patients with breast cancer. Journal of Clinical Oncology, 5(3), 348–353.
Levy, S. M., Herberman, R. B., Maluish, A. M., Schlien, B., & Lippman, M. (1985). Prognostic risk assessment in primary breast cancer by behavioral and immunological parameters. Health Psychology, 4(2), 99–113.
Seruga, B., Zhang, H., Bernstein, L. J., & Tannock, I. F. (2008). Cytokines and their relationship to the symptoms and outcome of cancer. Nature Reviews Cancer, 8(11), 887–899.
Kurzrock, R. (2001). The role of cytokines in cancer-related fatigue. Cancer, 92(6 Suppl), 1684–1688.
Menzies, H., Chochinov, H., & Breitbart, W. (2005). Cytokines, cancer, and depression: Connecting the dots [comment]. Journal of Supportive Oncology, 3, 55–57.
Quesada, J. R., Talpaz, M., Rios, A., Kurzrock, R., & Gutterman, J. U. (1986). Clinical toxicity of interferons in cancer patients: a review. Journal of Clinical Oncology, 4(2), 234–243.
Maes, M., Meltzer, H. Y., Bosmans, E., Bergmans, R., Vandoolaeghe, E., Ranjan, R., et al. (1995). Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. Journal of Affective Disorders, 34(4), 301–309.
Musselman, D. L., Miller, A. H., Porter, M. R., Manatunga, A., Gao, F., Penna, S., et al. (2001). Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. American Journal of Psychiatry, 158(8), 1252–1257.
Schubert, C., Hong, S., Natarajan, L., Mills, P. J., Dimsdale, J. E., Schubert, C., et al. (2007). The association between fatigue and inflammatory marker levels in cancer patients: A quantitative review. Brain, Behavior, and Immunity, 21(4), 413–427.
Ahlberg, K., Ekman, T., & Gaston-Johansson, F. (2004). Levels of fatigue compared to levels of cytokines and hemoglobin during pelvic radiotherapy: A pilot study. Biological Research Nursing, 5(3), 203–210.
Meyers, C. A., Albitar, M., Estey, E., Meyers, C. A., Albitar, M., & Estey, E. (2005). Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer, 104(4), 788–793.
Rich, T. A. (2007). Symptom clusters in cancer patients and their relation to EGFR ligand modulation of the circadian axis. The Journal of Supportive Oncology, 5(4), 167–174; discussion 176–177.
Bender, C. M., Sereika, S. M., Brufsky, A. M., Ryan, C. M., Vogel, V. G., Rastogi, P., et al. (2007). Memory impairments with adjuvant anastrozole versus tamoxifen in women with early-stage breast cancer. Menopause, 14(6), 995–998.
Posener, J. A., Schildkraut, J. J., Samson, J. A., & Schatzberg, A. F. (1996). Diurnal variation of plasma cortisol and homovanillic acid in healthy subjects. Psychoneuroendocrinology, 21(1), 33–38.
Bower, J. E., Ganz, P. A., Aziz, N., Bower, J. E., Ganz, P. A., & Aziz, N. (2005). Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosomatic Medicine, 67(2), 277–280.
Bailey, S. P., Davis, J. M., & Alhlborn, E. N. (1993). Neuroendocrine and substrate responses to altered brain 5-HT activity during prolonged exercise to fatigue. Journal of Applied Physiology, 74, 3006–3012.
Andrews, P. L. R., Morrow, G. R., & Hickok, J. T. (2004). Mechanisms and models of fatigue associated with cancer and its treatment. Evidence of pre-clinical and clinical studies. In J. Armes, M. Krishnasamy, & I. Higginson (Eds.), Fatigue in cancer (pp. 51–87). Oxford: Oxford University Press.
Roscoe, J. A., Morrow, G. R., Hickok, J. T., Mustian, K. M., Griggs, J. J., Matteson, S. E., et al. (2005). Effect of paroxetine hydrochloride (Paxil) on fatigue and depression in breast cancer patients receiving chemotherapy. Breast Cancer Research and Treatment, 89(3), 243–249.
Morrow, G. R., Hickok, J. T., Roscoe, J. A., Raubertas, R. F., Andrews, P. L., Flynn, P. J., et al. (2003). Differential effects of paroxetine on fatigue and depression: A randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Journal of Clinical Oncology, 21(24), 4635–4641.
Berger, A. M. (1998). Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncology Nursing Forum, 25(1), 51–62.
Mormont, M. C. (1997). Circadian-system alterations during cancer processes: A review. International Journal of Cancer, 70, 241–247.
Agteresch, H. J., Dagnelie, P. C., van der Gaast, A., Stijnen, T., & Wilson, J. H. (2000). Randomized clinical trial of adenosine 5′-triphosphate in patients with advanced non-small-cell lung cancer. Journal of the National Cancer Institute, 92(4), 321–328.
Segerstrom, S. C., & Miller, G. E. (2004). Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin, 130(4), 601–630.
Reyes-Gibby, C. C., Wu, X., Spitz, M., Kurzrock, R., Fisch, M., Bruera, E., et al. (2008). Molecular epidemiology, cancer-related symptoms, and cytokines pathway. Lancet Oncology, 9(8), 777–785.
Reyes-Gibby, C. C., Spitz, M., Wu, X., Merriman, K., Etzel, C., Bruera, E., et al. (2007). Cytokine genes and pain severity in lung cancer: Exploring the influence of TNF-alpha-308 G/A IL6–174G/C and IL8–251T/A. Cancer Epidemiology, Biomarkers and Prevention, 16(12), 2745–2751.
Irwin, M. R., Miller, A. H., Irwin, M. R., & Miller, A. H. (2007). Depressive disorders and immunity: 20 years of progress and discovery. Brain, Behavior, and Immunity, 21(4), 374–383.
Collado-Hidalgo, A., Bower, J. E., Ganz, P. A., Irwin, M. R., & Cole, S. W. (2008). Cytokine gene polymorphisms and fatigue in breast cancer survivors: Early findings. Brain, Behavior, and Immunity, 22, 1197–1200.
Shafqat, A., Einhorn, L. H., Hanna, N., Sledge, G. W., Hanna, A., Juliar, B. E., et al. (2005). Screening studies for fatigue and laboratory correlates in cancer patients undergoing treatment. Annals of Oncology, 16(9), 1545–1550.
Whistler, T., Taylor, R., Craddock, R. C., Broderick, G., Klimas, N., Unger, E. R., et al. (2006). Gene expression correlates of unexplained fatigue. Pharmacogenomics, 7(3), 395–405.
Aspler, A. L., Bolshin, C., Vernon, S. D., Broderick, G., Aspler, A. L., Bolshin, C., et al. (2008). Evidence of inflammatory immune signaling in chronic fatigue syndrome: A pilot study of gene expression in peripheral blood. Behavioral and Brain Functions Electronic Resource: BBF, 4, 44.
Kerr, J. R., Burke, B., Petty, R., Gough, J., Fear, D., Mattey, D. L., et al. (2008). Seven genomic subtypes of chronic fatigue syndrome/myalgic encephalomyelitis: A detailed analysis of gene networks and clinical phenotypes. Journal of Clinical Pathology, 61(6), 730–739.
Reyes, M., Nisenbaum, R., Hoaglin, D. C., Unger, E. R., Emmons, C., Randall, B., et al. (2003). Prevalence and incidence of chronic fatigue syndrome in Wichita, Kansas. Archives of Internal Medicine, 163(13), 1530–1536.
Plomin, R., DeFries, J. C., McClearn, G. E., & McGuffin, P. (2001). Behavioral Genetics. New York: Worth Publishers and W. H. Freeman and Co.
Hartyl, D. L., & Jones, E. W. (Eds.). (2009). Genetics: Analysis of genes, genomes (7th ed.). Sudbury, Massachusetts: Jones and Bartlett.
Acknowledgments
GENEQOL Consortium participants per March 2009: Amy P. Abertnethy, Duke Cancer Care Research Program, Duke University Medical Center, Durham, NC, US; Frank Baas, Laboratory of Neurogenetics, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands; Andrea M. Barsevick, Cancer Prevention and Control Program, Fox Chase Cancer Center, Philadelphia, PA, US; Meike Bartels, Department of Biological Psychology, VU University, Amsterdam, the Netherlands; Dorret I. Boomsma, Department of Biological Psychology, VU University, Amsterdam, the Netherlands; Cynthia Chauhan, Cancer Advocay, Wichita, KS, US; Charles S. Cleeland, Department of Symptom Research, The University of Texas M. D. Anderson Cancer Center, Houston, TX, US; Amylou C. Dueck, Section of Biostatistics, Mayo Clinic, Scottsdale, AZ, US; Marlene H. Frost, Women’s Cancer Program, Mayo Clinic, Rochester, MN, US; Per Hall, Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden; Michele Y. Halyard, Department of Radiation Oncology, Mayo Clinic, Scottsdale, AZ, US; Pål Klepstad, Department of Intensive Care Medicine, St Olavs University Hospital, Norwegian University of Technology and Science, Trondheim, Norway; Nicholas G. Martin, Queensland Institute of Medical Research, Brisbane, Australia; Christine Miaskowski, School of Nursing, University of California, San Francisco, CA, US; Miriam Mosing, Queensland Institute of Medical Research, Brisbane, Australia; Benjamin Movsas, Department of Radiation Oncology, Henry Ford Health System, Detroit, MI, US; Cornelis J. F. Van Noorden, Department of Cell Biology and Histology, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands; Donald L. Patrick, Department of Health Services, University of Washington, Seattle, WA, US; Nancy L. Pedersen, Department of Medical Epidemiology and Biostatistics, Karolinska; Institute, Stockholm, Sweden; Mary E. Ropka, Cancer Prevention and Control Program, Fox Chase Cancer Center, Cheltenham, PA, US; Quiling Shi, Department of Symptom Research, The University of Texas M. D. Anderson Cancer Center, Houston, TX, US; Gen Shinozaki, Department of Psychiatry and Psychology, Mayo Clinic, Rochester, MN, US; Jasvinder A. Singh, Minneapolis Veterans Affairs Medical Center and University of Minnesota, Minneapolis, MN and Mayo Clinic College of Medicine, Rochester, MN, US; Jeff A. Sloan, Department of Health Sciences Research, Mayo Clinic, Rochester, MN, US; Mirjam A. G. Sprangers, Department of Medical Psychology, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands; Ruut Veenhoven, Faculty of Social Sciences, Erasmus University Rotterdam, Rotterdam, The Netherlands; Ping Yang, Department of Genetic Epidemiology, Mayo Clinic, Rochester, MN, US; Aeilko H. Zwinderman, Department of Clinical Epidemiology and Biostatistics, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands.
Author information
Authors and Affiliations
Consortia
Corresponding author
Appendix: Glossary [63, 64]
Appendix: Glossary [63, 64]
-
Case Definition: Criteria or standards used to identify an instance of a disease, syndrome, or condition;
-
DNA (deoxyribonucleic acid): The two-stranded molecule that encodes genetic information;
-
Gene: The basic unit of inheritance. A sequence of DNA bases that codes for a particular product;
-
Genome: All the DNA sequences of an organism;
-
Genotype: The genetic constitution of an organism that is not manifested as outward characteristics;
-
Phenotype: Physical characteristics of an organism or the presence of a disease that may or may not be genetic;
-
Polymorphism: A locus with two or more alleles (alternative forms of a gene at a locus);
-
Functional polymorphism: DNA sequence variations that alter the expression and/or functioning of the gene product;
-
Single nucleotide polymorphism (SNP): Sequences in the genome that differ by a single nucleotide between one portion of the population and another.
Rights and permissions
About this article
Cite this article
Barsevick, A., Frost, M., Zwinderman, A. et al. I’m so tired: biological and genetic mechanisms of cancer-related fatigue. Qual Life Res 19, 1419–1427 (2010). https://doi.org/10.1007/s11136-010-9757-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-010-9757-7