ABSTRACT
Purpose
The narrow efficacy-toxicity window of anticancer agents necessitates understanding of factors contributing to their disposition. This is especially true for camptothecins as they exist in the lactone and carboxylate forms with each moiety differentially interacting with efflux or uptake transporters. Here we determined the disposition of the lactone and carboxylate forms of AR-67, a 3rd generation camptothecin analogue.
Methods
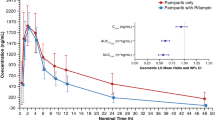
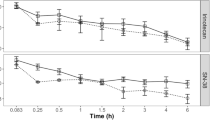
Pharmacokinetic studies were conducted in rats given intravenous boluses of AR-67 lactone or carboxylate with or without pharmacologic inhibitor pretreatment (GF120918 or rifampin). Pharmacokinetic modeling was used to estimate clearances, while simulations assessed the impact of clearance changes on overall AR-67 exposure.
Results
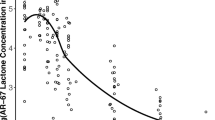
Our modeling showed that carboxylate clearance was 3.5-fold higher than that of the lactone. GF120918 decreased lactone clearance only, but rifampin decreased both lactone and carboxylate clearances. Simulations showed that decreasing carboxylate clearance, which controls the overall drug disposition, leads to significant increase in AR-67 exposure.
Conclusion
The apparent in vivo blood stability of AR-67 is partly dependent on the increased carboxylate clearance. This may have clinical implications for populations with single nucleotide polymorphisms that impair the function of uptake transporter genes (e.g., SLCO1B1), which are potentially responsible for AR-67 carboxylate clearance.





Similar content being viewed by others
Abbreviations
- AUC:
-
area under the plasma concentration versus time curve
- BCRP/bcrp:
-
breast cancer resistance protein
- OATP/Oatp:
-
organic anion transporting polypeptide
- P-gp:
-
p-glycoprotein
- USP:
-
United States Pharmacopeia
REFERENCES
Bom D, Curran DP, Kruszewski S, Zimmer SG, Thompson Strode J, Kohlhagen G et al. The novel silatecan 7-tert-butyldimethylsilyl-10-hydroxycamptothecin displays high lipophilicity, improved human blood stability, and potent anticancer activity. J Med Chem. 2000;43:3970–80.
Pollack IF, Erff M, Bom D, Burke TG, Strode JT, Curran DP. Potent topoisomerase I inhibition by novel silatecans eliminates glioma proliferation in vitro and in vivo. Cancer Res. 1999;59:4898–905.
Arnold SM, Rinehart JJ, Tsakalozou E, Eckardt JR, Fields SZ, Shelton BJ et al. A phase I study of 7-t-butyldimethylsilyl-10-hydroxycamptothecin in adult patients with refractory or metastatic solid malignancies. Clin Cancer Res. 2010;16:673–80.
Fassberg J, Stella VJ. A kinetic and mechanistic study of the hydrolysis of camptothecin and some analogues. J Pharm Sci. 1992;81:676–84.
Jaxel C, Kohn KW, Wani MC, Wall ME, Pommier Y. Structure-activity study of the actions of camptothecin derivatives on mammalian topoisomerase I: evidence for a specific receptor site and a relation to antitumor activity. Cancer Res. 1989;49:1465–9.
Hsiang YH, Liu LF. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988;48:1722–6.
Burke TG, Mi Z. Preferential binding of the carboxylate form of camptothecin by human serum albumin. Anal Biochem. 1993;212:285–7.
Liehr JG, Ahmed AE, Giovanella BC. Pharmacokinetics of camptothecins administered orally. Ann N Y Acad Sci. 1996;803:157–63.
Creaven PJ, Allen LM, Muggia FM. Plasma camptothecin (NSC-100880) levels during a 5-day course of treatment: relation to dose and toxicity. Cancer Chemother Rep. 1972;56:573–8.
Natelson EA, Giovanella BC, Verschraegen CF, Fehir KM, De Ipolyi PD, Harris N et al. Phase I clinical and pharmacological studies of 20-(S)-camptothecin and 20-(S)-9-nitrocamptothecin as anticancer agents. Ann N Y Acad Sci. 1996;803:224–30.
Lalloo AK, Luo FR, Guo A, Paranjpe PV, Lee SH, Vyas V et al. Membrane transport of camptothecin: facilitation by human P-glycoprotein (ABCB1) and multidrug resistance protein 2 (ABCC2). BMC Med. 2004;2:16.
Nozawa T, Minami H, Sugiura S, Tsuji A, Tamai I. Role of organic anion transporter OATP1B1 (OATP-C) in hepatic uptake of irinotecan and its active metabolite, 7-ethyl-10-hydroxycamptothecin: in vitro evidence and effect of single nucleotide polymorphisms. Drug Metab Dispos. 2005;33:434–9.
Marchetti S, Oostendorp RL, Pluim D, van Eijndhoven M, van Tellingen O, Schinkel AH et al. In vitro transport of gimatecan (7-t-butoxyiminomethylcamptothecin) by breast cancer resistance protein, P-glycoprotein, and multidrug resistance protein 2. Mol Cancer Ther. 2007;6:3307–13.
Li H, Jin HE, Kim W, Han YH, Kim DD, Chung SJ et al. Involvement of P-glycoprotein, multidrug resistance protein 2 and breast cancer resistance protein in the transport of belotecan and topotecan in Caco-2 and MDCKII cells. Pharm Res. 2008;25:2601–12.
Adane ED, Monks N, Roberts MJ, Horn J, Moscow JA, Leggas M. Transporter Pathways for the Novel Camptothecin Analog DB-67. AAPS Abstract M1032, AAPS Workshop on Drug Transporters in ADME: From the Bench to the Bedside (2007).
Scott DO, Bindra DS, Stella VJ. Plasma pharmacokinetics of lactone and carboxylate forms of 20(S)-camptothecin in anesthetized rats. Pharm Res. 1993;10:1451–7.
Scott DO, Bindra DS, Sutton SC, Stella VJ. Urinary and biliary disposition of the lactone and carboxylate forms of 20(S)-camptothecin in rats. Drug Metab Dispos. 1994;22:438–42.
Davies BE, Minthorn EA, Dennis MJ, Rosing H, Beijnen JH. The pharmacokinetics of topotecan and its carboxylate form following separate intravenous administration to the dog. Pharm Res. 1997;14:1461–5.
Rivory LP, Chatelut E, Canal P, Mathieu-Boue A, Robert J. Kinetics of the in vivo interconversion of the carboxylate and lactone forms of irinotecan (CPT-11) and of its metabolite SN-38 in patients. Cancer Res. 1994;54:6330–3.
Ebling WF, Jusko WJ. The determination of essential clearance, volume, and residence time parameters of recirculating metabolic systems: the reversible metabolism of methylprednisolone and methylprednisone in rabbits. J Pharmacokinet Biopharm. 1986;14:557–99.
Cheng HY, Jusko WJ. Mean residence times and distribution volumes for drugs undergoing linear reversible metabolism and tissue distribution and linear or nonlinear elimination from the central compartments. Pharm Res. 1991;8:508–11.
Horn J, Jordan SL, Song L, Roberts MJ, Anderson BD, Leggas M. Validation of an HPLC method for analysis of DB-67 and its water soluble prodrug in mouse plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;844:15–22.
D’Argenio DZ, Schumitzky A. ADAPT II User’s Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Biomedical Simulations Resource (1997).
Cheng H, Jusko WJ. Pharmacokinetics of reversible metabolic systems. Biopharm Drug Dispos. 1993;14:721–66.
Rowland M, Tozer TN. Clinical Pharmacokinetics: Concents and Applications, Williams & Wilkins (1995).
Mi Z, Burke TG. Differential interactions of camptothecin lactone and carboxylate forms with human blood components. Biochemistry. 1994;33:10325–36.
Jonker JW, Smit JW, Brinkhuis RF, Maliepaard M, Beijnen JH, Schellens JH et al. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst. 2000;92:1651–6.
Kruijtzer CM, Beijnen JH, Rosing H, ten Bokkel Huinink WW, Schot M, Jewell RC et al. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J Clin Oncol. 2002;20:2943–50.
Kuppens IE, Witteveen EO, Jewell RC, Radema SA, Paul EM, Mangum SG et al. A phase I, randomized, open-label, parallel-cohort, dose-finding study of elacridar (GF120918) and oral topotecan in cancer patients. Clin Cancer Res. 2007;13:3276–85.
Oostendorp RL, van de Steeg E, van der Kruijssen CM, Beijnen JH, Kenworthy KE, Schinkel AH et al. Organic anion-transporting polypeptide 1B1 mediates transport of Gimatecan and BNP1350 and can be inhibited by several classic ATP-binding cassette (ABC) B1 and/or ABCG2 inhibitors. Drug Metab Dispos. 2009;37:917–23.
Lau YY, Okochi H, Huang Y, Benet LZ. Pharmacokinetics of atorvastatin and its hydroxy metabolites in rats and the effects of concomitant rifampicin single doses: relevance of first-pass effect from hepatic uptake transporters, and intestinal and hepatic metabolism. Drug Metab Dispos. 2006;34:1175–81.
Zong J, Pollack GM. Modulation of P-glycoprotein transport activity in the mouse blood-brain barrier by rifampin. J Pharmacol Exp Ther. 2003;306:556–62.
Sparreboom A, Gelderblom H, Marsh S, Ahluwalia R, Obach R, Principe P et al. Diflomotecan pharmacokinetics in relation to ABCG2 421C>A genotype. Clin Pharmacol Ther. 2004;76:38–44.
Niemi M, Schaeffeler E, Lang T, Fromm MF, Neuvonen M, Kyrklund C et al. High plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1). Pharmacogenetics. 2004;14:429–40.
Lau YY, Huang Y, Frassetto L, Benet LZ. effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther. 2007;81:194–204.
Lau YY, Okochi H, Huang Y, Benet LZ. Multiple transporters affect the disposition of atorvastatin and its two active hydroxy metabolites: application of in vitro and ex situ systems. J Pharmacol Exp Ther. 2006;316:762–71.
ACKNOWLEDGMENTS
This work was supported in part by the National Institutes of Health (RCA123867A) and research grants from Arno Therapeutics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adane, E.D., Liu, Z., Xiang, TX. et al. Factors Affecting the In Vivo Lactone Stability and Systemic Clearance of the Lipophilic Camptothecin Analogue AR-67. Pharm Res 27, 1416–1425 (2010). https://doi.org/10.1007/s11095-010-0137-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0137-3