Abstract
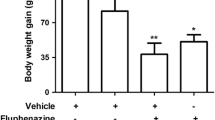
Classical antipsychotics can produce motor disturbances like tardive dyskinesia in humans and orofacial dyskinesia in rodents. These motor side effects have been associated with oxidative stress production in specific brain areas. Thus, some studies have proposed the use of natural compounds with antioxidant properties against involuntary movements induced by antipsychotics. Here, we examined the possible antioxidant activity of Bauhinia forficata (B. forficata), a plant used in folk medicine as a hypoglycemic, on brain lipid peroxidation induced by different pro-oxidants. B. forficata prevented the formation of lipid peroxidation induced by both pro-oxidants tested. However, it was effective against lipid peroxidation induced by sodium nitroprusside (IC50 = 12.08 μg/mL) and Fe2+/EDTA (IC50 = 41.19 μg/mL). Moreover, the effects of B. forficata were analyzed on an animal model of orofacial dyskinesia induced by long-term treatment with haloperidol, where rats received haloperidol each 28 days (38 mg/kg) and/or B. forficata decoction daily (2.5 g/L) for 16 weeks. Vacuous chewing movements (VCMs), locomotor and exploratory activities were evaluated. Haloperidol treatment induced VCMs, and co-treatment with B. forficata partially prevented this effect. Haloperidol reduced the locomotor and exploratory activities of animals in the open field test, which was not modified by B. forficata treatment. Our present data showed that B. forficata has antioxidant potential and partially protects against VCMs induced by haloperidol in rats. Taken together, our data suggest the protection by natural compounds against VCMs induced by haloperidol in rats.





Similar content being viewed by others
References
Andreassen OA, Jorgensen HA (2000) Neurotoxicity associated with neurolepticinduced oral dyskinesias in rats Implications for tardive dyskinesia. Prog Neurobiol 61:525–541. doi:10.1016/S0301-0082(99)00064-7
Kane JM, Smith JM (1982) Tardive dyskinesia: prevalence and risk factors, 1959 to 1979. Arch Gen Psychiatry 39:473–481. doi:10.1001/archpsyc.1982.04290040069010
Woerner MG, Kane JM, Lieberman JA, Alvir J, Bergmann KJ, Borenstein M et al (1991) The prevalence of tardive dyskinesia. J Clin Psychopharmacol 11:34–42
Yassa R, Jeste DV (1992) Gender differences in tardive dyskinesia: a critical review of the literature. Schizophr Bull 18:701–715. doi:10.1093/schbul/18.4.701
Casey DE (1985) Tardive dyskinesia: reversible and irreversible. Psychopharmacology 2:88–97
Crane GE (1973) Persistent dyskinesia. Br J Psychiatry 122:395–405. doi:10.1192/bjp.122.4.395
Jeste DV, Potkin SG, Sinha S, Feder S, Wyatt RJ (1979) Tardive dyskinesia- reversible and persistent. Arch Gen Psychiatry 36:585–590. doi:10.1001/archpsyc.1979.01780050095012
Rogoza RM, Fairfax DF, Henry P, Marandi S, Khan RF, Gupta SK, Mishra RK (2004) Electron spin resonance spectroscopy reveals alpha-phenyl-N-tert-butylnitrone spin-traps free radicals in rat striatum and prevents haloperidol-induced vacuous chewing movements in the rat model of human tardive dyskinesia. Synapse 54:156–163. doi:10.1002/syn.20078
Seeman P, Van Tol HH (1994) Dopamine receptor pharmacology. Trends Pharmacol Sci 15:264–270. doi:10.1016/0165-6147(94)90323-9
Sookram C, Tan M, Daya R, Heffernan S, Mishra RK (2011) Curcumin prevents haloperidol-induced development of abnormal oro-facial movements: possible implications of Bcl-XL in its mechanism of action. Synapse 65:788–794. doi:10.1002/syn.20905
Berg D, Youdim MB, Riederer P (2004) Redox imbalance. Cell Tissue Res 318:201–213. doi:10.1007/s00441-004-0976-5
Kohen R, Nyska A (2002) Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30:620–650. doi:10.1080/01926230290166724
Dwork AJ, Lawler G, Zybert PA, Durkin M, Osman M, Willson N, Barkai AI (1990) An autoradiographic study of the uptake and distribution of iron by the brain of the young rat. Brain Res 518:31–39. doi:10.1016/0006-8993(90)90950-G
Que EL, Domaille DW, Chang CJ (2008) Metals in Neurobiology: Probing Their Chemistry and Biology with Molecular Imaging. Chem Rev 108:1517–1549. doi:10.1021/cr078203u
Fang J, McKay G, Song JX, Remillrd A, Li XM, Midha K (2001) In vitro characterization of the metabolism of haloperidol using recombinant cytochrome P450 enzymes and human liver microsomes. Drug Metab Dispos 29:1638–1643. doi:0090-9556/01/1229
Wright AM, Bempong J, Kirby ML, Barlow RL, Bloomquist JR (1998) Effects of haloperidol metabolites on neurotransmitter uptake and release: possible role in neurotoxicity and tardive dyskinesia. Brain Res 788:215–222. doi:10.1016/S0006-8993(97)01551-5
Polydoro M, Schroder N, Lima MNM, Caldana F, Laranja DC, Bromberg E, Roesler R, Quevedo J, Moreira JCF, Dal-Pizzol F (2004) Haloperidol- and clozapine-induced oxidative stress in the rat brain. Pharmacol Biochem Behav 78:751–756. doi:10.1016/j.pbb.2004.05.018
Burger ME, Fachinetto R, Zeni G, Rocha JBT (2005) Ebselen attenuates haloperidol-induced orofacial dyskinesia and oxidative stress in rat brain. Pharmacol Biochem Behav 81:608–615. doi:10.1016/j.pbb.2005.05.002
Fachinetto R, Burger ME, Wagner C, Wondracek DC, Brito VB, Nogueira CW, Ferreira J, Rocha JBT (2005) High fat diet increases the incidence of orofacial dyskinesia and oxidative stress in specific brain regions of rats. Pharmacol Biochem Behav 81:585–592. doi:10.1016/j.pbb.2005.05.001
Harvey BH, Joubert C, Preez JL, Berk M (2008) Effect of Chronic N-Acetyl Cysteine Administration on Oxidative Status in the Presence and Absence of Induced Oxidative Stress in Rat Striatum. Neurochem Res 33:508–517. doi:10.1007/s11064-007-9466-y
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477. doi:10.1021/np068054v
Pereira RP, Fachinetto R, Prestes AS, Puntel RL, Silva GNS et al (2009) Antioxidant Effects of Different Extracts from Melissa officinalis, Matricaria recutita and Cymbopogon citrates. Neurochem Res 34:973–983. doi:10.1007/s11064-008-9861-z
Sudati JH, Fachinetto R, Pereira RP, Boligon AA, Athayde ML et al (2009) In vitro Antioxidant Activity of Valeriana officinalis Against Different Neurotoxic Agents. Neurochem Res 34:1372–1379. doi:10.1007/s11064-009-9917-8
Busanello A, Barbosa NBV, Peroza LR, Farias LE, Burger ME, Barreto KP, Fachinetto R (2011) Resveratrol protects against a model of vacuous chewing movements induced by reserpine in mice. Behav Pharmacol 22:71–75. doi:10.1097/FBP.0b013e328341e9b4
Busanello A, Peroza LR, Wagner C, Sudati JH, Pereira RP, Prestes AS, Rocha JBT, Fachinetto R, Barbosa NBV (2012) Resveratrol reduces vacuous chewing movements induced by acute treatment with fluphenazine Pharmacol. Biochem and Behav 101:307–310. doi:10.1016/j.pbb.2012.01.007
Pereira RP, Fachinetto R, Prestes AS, Wagner C, Sudati JH, Boligon AA, Athayde ML, Morsch VM, Rocha JBT (2011) Valeriana officinalis ameliorates vacuous chewing movements induced by reserpine in rats. J Neural Tansm 118:1547–1557. doi:10.1007/s00702-011-0640-7
Reckziegel P, Peroza LR, Schaffer LF, Ferrari MC, Freitas CM, Burger ME, Fachinetto R (2013) Gallic acid decreases vacuous chewing movements induced by reserpine in rats. Pharmacol Biochem Behav, accepted manuscript. doi:10.1016/j.pbb.2013.01.001
Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS (2003) Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care 26:1277–1294. doi:10.2337/diacare.26.4.1277
Amaral ACF, Simões EV, Ferreira JLP (2005) Coletânea Científica de Plantas de Uso Medicinal. FIOCRUZ, Rio de Janeiro
Mali RG, Mahajan SG, Mehta AA (2007) Rakta Kanchan (Bauhinia variegata): chemistry, traditional and medicinal uses—a review. Pharmacogn Rev 1:314–319
Damasceno DC, Volpato GT, Calderon IM, Aguilar R, Rudge MV (2004) Effect of Bauhinia forficata extract in diabetic pregnant rats: maternal repercussions. Phytomedicine 11:196–201. doi:10.1078/0944-7113-00348
de Sousa E, Zanatta L, Seifriz I et al (2004) Hypoglycemic effect and antioxidant potential of kaempferol-3,7-O-(alpha)-dirhamnoside from Bauhinia forficata leaves. J Nat Prod 67:829–832. doi:10.1021/np030513u
Khalil NM, Pepato MT, Brunetti IL (2008) Free radical scavenging profile and myeloperoxidase inhibition of extracts from antidiabetic plants: Bauhinia forficata and Cissus sicyoides. Biol Res 41:165–171. doi:10.4067/S0716-97602008000200006
Silva EG, Behr GA, Zanotto-Filho A, Lorenzi R, Pasquali MAB, Ravazolo LG et al (2007) Antioxidant activities and free radical scavenging potential of Bauhinia microstachya (RADDI) MACBR. (Caesalpinaceae) extracts linked to their polyphenol content. Biol Pharm Bull 30:1488–1496. doi:10.1248/bpb.30.1488
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Meth Enzymol 299:152–178. doi:10.1016/S0076-6879(99)99017-1
Ohkawa H, Ohishi Ν, Yagi Κ (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Wagner C, Fachinetto R, Dalla Corte CL, Brito VB, Severo D, Dias GOC, Morel AF, Nogueira CW, Rocha JBT (2006) Quercitrin, a glycoside form of quercetin, prevents lipid peroxidation in vitro. Brain Res 1107:192–198. doi:10.1016/j.brainres.2006.05.084
Fachinetto R, Villarinho JG, Wagner C, Pereira RP, Ávila DS, Burger ME et al (2007) Valeriana officinalis does not alter the orofacial dyskinesia induced by haloperidol in rats: role of dopamine transporter. Prog Neuropsychopharmacol Biol Psychiatry 31:1478–1486. doi:10.1016/j.pnpbp.2007.06.028
Broadhurst PL (1960) Experiments in psychogenetics. In: Eysenk HJ (ed) Experiments in personality. Routledge & Kegan Paul, London
Guoa M, Pereza C, Weia Y, Rapozaa E, Sua G, Bou-Abdallahb F, Chasteenb ND (2007) Iron-binding properties of plant phenolics and cranberry’s bioeffects. Dalton Trans 43:4951–4961
Halliwell B, Gutteridge JMC (1985) Oxygen radicals and the nervous system. Trends Neurosci 8:22–26. doi:10.1016/0166-2236(85)90010-4
Cohen G (1988) Oxygen radicals and Parkinson’s disease. In: Halliwell B (ed) Oxygen radicals and tissue injury. FASEB, Bethesda, pp 130–135
Bates JN, Baker MT, Guerra R, Harrison DG (1991) Nitric oxide generation from nitroprusside by vascular tissue. Biochem Pharmacol 42(Suppl):S157–S165. doi:10.1016/0006-2952(91)90406-U
Chen J, Chang B, Williams M, Murad F (1991) Sodium nitroprusside degenerates cultured rat striatal neurons. NeuroReport 2:121–123. doi:10.1097/00001756-199103000-00002
Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH (1991) Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci USA 88:6368–6371
Rauhala P, KhaldiI A, Mohanakumar KP, Chiueh CC (1998) Apparent role of hydroxyl radicals in oxidative brain injury induced by sodium nitroprusside. Free Radic Biol Med 24:1065–1073. doi:10.1016/S0891-5849(97)00386-9
Graf E, Mahoney JR, Bryant RG, Eaton JW (1984) Iron catalyzed hydroxyl radical formation: stringent requirement for free iron coordination site. J Biol Chem 259:3620–3624
Halliwell B, Gutteridge JMC (1989) Free radicals in biology and medicine. Clarendon Press, Oxford
Dean RT, Fu S, Stocker R, Davies MJ (1997) Biochemistry and pathology of radical-mediated protein oxidation. Biochem J 324:1–18. doi:978-0-19-850097-1
Lerner P, Nose P, Gordon EK, Lovenberg W (1977) Haloperidol: effect of long-term treatment on rat striatal dopamine synthesis and turnover. Science 197:181–183
Mariani E, Polidori MC, Cherubini A et al (2005) Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Analyt Technol Biomed Life Sci 827:65–75. doi:10.1016/j.jchromb.2005.04.023
Abílio VC, Silva RH, Carvalho RC, Grassl C, Calzavara MB, Registro S et al (2004) Important role of striatal catalase in aging- and reserpine-induced oral dyskinesia. Neuropharmacology 47:263–272. doi:10.1016/j.neuropharm.2004.04.003
Andreassen OA, Ferrante RJ, Aamo TO, Beal MF, Jorgensen HA (2003) Oral dyskinesias and histopathological alterations in substantia nigra after long-term haloperidol treatment of old rats. Neuroscience 122:717–725. doi:10.1016/j.neuroscience.2003.08.058
Burger ME, Fachineto R, Alves A, Callegari L, Rocha JB (2005) Acute reserpine and subchronic haloperidol treatments change synaptosomal brain glutamate uptake and elicit orofacial dyskinesia in rats. Brain Res 1031:202–210. doi:10.1016/j.brainres.2004.10.038
Faria RR, Abilio VC, Grassl C, Chinen CC, Negrão LTR, de Castro JPMV et al (2005) Beneficial effects of vitamin C and vitamin E on reserpine-induced oral dyskinesis in rats: critical role of striatal catalase activity. Neuropharmacology 48:993–1001. doi:10.1016/j.neuropharm.2005.01.014
Naidu PS, Singh A, Kulkarni SK (2003) Quercetin, a bioflavonoid, attenuates haloperidolinduced orofacial dyskinesia. Neuropharmacology 44:1100–1106. doi:10.1016/S0028-3908(03)00101-1
Colpo G, Trevisol F, Teixeira AM, Fachinetto R, Pereira RP, Athayde ML et al (2007) Ilex paraguariensis has antioxidant potential and attenuates haloperidol-induced orofacial dyskinesia and memory dysfunction in rats. Neurotox Res 12:171–180. doi:10.1007/BF03033914
Fachinetto R, Villarinho JG, Wagner C, Pereira RP, Puntel RL, Paixão MW et al (2007) Diphenyl diselenide decreases the prevalence of vacuous chewing movements induced by fluphenazine in rats. Psychopharmacology 194:423–432. doi:10.1007/s00213-007-0831-y
Lazzarini M, Salum C, Del Bel EA (2005) Combined treatment of ascorbic acid or alpha-tocopherol with dopamine receptor antagonist or nitric oxide synthase inhibitor potentiates cataleptic effect in mice. Psychopharmacology 181:71–79. doi:10.1007/s00213-005-2222-6
Sachdev P, Saharov T, Cathcart S (1999) The preventative role of antioxidants (selegiline and vitamin E) in a rat model of tardive dyskinesia. Biol Psychiatry 46:1672–1681. doi:10.1016/S0006-3223(99)00091-8
Eranti VS, Gangadhar BN, Janakiramaiah N (1998) Haloperidol-induced extrapyramidal reaction: lack of protective effect by vitamin E. Psychopharmacology 140:418–420. doi:10.1007/s002130050784
Pham DQ, Plakogiannis R (2005) Vitamin E supplementation in Alzheimer’s disease, Parkinson’s disease, tardive dyskinesia, and cataract: part 2. Ann Pharmacother 39:2065–2072. doi:10.1345/aph.1G271
Kelley AE, Bakshi VP, Delfs JM, Lang CG (1989) Cholinergic stimulation of the ventrolateral striatum elicits mouth movements in rats: pharmacological and regional specificity. Psychopharmacology 99:542–549. doi:10.1007/BF00589906
Salamone JD, Ishiwari K, Betz AJ, Farrar AM, Mingote SM, Font L et al (2008) Dopamine/adenosine interactions related to locomotion and tremor in animal models: possible relevance to parkinsonism. Parkinsonism Relat Disord 2(Suppl):S130–S134. doi:10.1016/j.parkreldis.2008.04.017
Acknowledgments
Financial support by FAPERGS (0904348-ARD-03/2009), CAPES, CNPq (473365/2009-0) and PIBIC, FINEP research grant ‘Rede Instituto Brasileiro de Neurociência (IBN-Net)’ (01.06.0842-00) is gratefully acknowledged. M.L. is recipient of PROBIC/FAPERGS and C.Q.L is recipient of PIBIC/CNPq fellowships. The authors are grateful to Dr. João Batista Teixeira da Rocha for his important contribution.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peroza, L.R., Busanello, A., Leal, C.Q. et al. Bauhinia forficata Prevents Vacuous Chewing Movements Induced by Haloperidol in Rats and Has Antioxidant Potential In Vitro. Neurochem Res 38, 789–796 (2013). https://doi.org/10.1007/s11064-013-0981-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-0981-8