Abstract
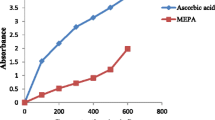
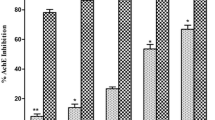
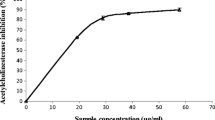
This study sought to investigate and compare the interaction of caffeic acid and chlorogenic acid on acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), and some pro-oxidants (FeSO4, sodium nitroprusside and quinolinic acid) induced oxidative stress in rat brain in vitro. The result revealed that caffeic acid and chlorogenic acid inhibited AChE and BChE activities in dose-dependent manner; however, caffeic acid had a higher inhibitory effect on AChE and BChE activities than chlorogenic acid. Combination of the phenolic acids inhibited AChE and BChE activities antagonistically. Furthermore, pro-oxidants such as, FeSO4, sodium nitroprusside and quinolinic acid caused increase in the malondialdehyde (MDA) contents of the brain which was significantly decreased dose-dependently by the phenolic acids. Inhibition of AChE and BChE activities slows down acetylcholine and butyrylcholine breakdown in the brain. Therefore, one possible mechanism through which the phenolic acids exert their neuroprotective properties is by inhibiting AChE and BChE activities as well as preventing oxidative stress-induced neurodegeneration. However, esterification of caffeic acid with quinic acid producing chlorogenic acid affects these neuroprotective properties.






Similar content being viewed by others
References
Pratico D, Delanty N (2000) Oxidative injury in diseases of the central nervous system: focus on Alzheimer’s disease. Am J Med 109:577–585
Marksberry WR, Lovell MA (2007) Damage to lipids, proteins, DNA and RNA in mild cognitive impairment. Arch Neurol 64:954–956
Oboh G, Rocha JBT (2007) Distribution and antioxidant activity of polyphenols in ripe and unripe tree pepper (Capsicum pubescens). J Food Biochem 31:456–473
Fraga CG, Oteiza PI, Golub MS, Gershwin ME, Keen CL (1990) Effect of aluminum on brain lipid peroxidation. Toxicol Lett 51:213–219
Stacey NH, Kappus H (1982) Cellular toxicity and lipid peroxidation in response to mercury. Toxicol Appl Pharmacol 63:29–35
Ehmann WD, Markesbery WR, Alauddin M (1986) Brain trace elements in Alzheimer’s disease. Neurotoxicology 7:195–206
Zago MP, Verstraeten SV, Oteiza PI (2000) Zinc in the prevention of Fe2+ initiated lipid and protein oxidation. Biol Res 33:143–150
Arnold SE, Kumar A (1993) Reversible dementias. Med Clin North Am 77(215):230
Orhan I, Sener B, Choudhary MI, Khalid A (2004) Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. J Ethnopharmacol 91:57–60
Schnider A (2001) Spontaneous confabulation, reality monitoring and the limbic system—a review. Brain Res 36:150–160
Brimijoin S (1983) Molecular forms of acetylcholinesterase in brain, nerve and muscle: nature, localization and dynamics. Prog Neurobiol 21:291–322
Conforti F, Statti GA, Menichini F (2007) Chemical and biological variability of hot pepper fruits (Capsicum annuum var. acuminatum L.) In relation to maturity stage. Food Chem 102:1096–1104
Ferrerira A, Proenc C, Serralheiro MLM, Arajo MEM (2006) The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J Ethnopharmacol 108:31–37
Clifford MN (1999) Chlorogenic acids and other cinnamatessnature, occurrence and dietary burden. J Sci Food Agric 79:362–372
Olthof MR, Hollman PCH, Katan MB (2001) Chlorogenic acid and caffeic acid are absorbed in humans. J Nutr 131:66–71
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Rad Biol Med 20:933–956
Park RM, Schulte PA, Bowman JD, Walker JT, Bondy SC, Yost MG, Touchstone JA, Dosemeci M (2005) Potential occupational risks for neurodegenerative diseases. Am J Ind Med 48(1):63–77
Tanaka T, Kojima T, Kawamori T, Wang A, Suzui M, Okamoto K, Mori H (1993) Inhibition of 4-nitroquinoline-1-oxide-induced rat tongue carcinogenesis by naturally occurring plant phenolics caffeic, ellagic, chlorogenic and ferulic acids. Carcinogen 14:1321–1325
Michaluart P, Masferrer JL, Carothers AM, Subbaramaiah K, Zweifel BS, Koboldt C, Mestre JR, Grunberger D, Sacks PG, Tanabe T (1999) Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res 59:2347–2352
Yan JJ, Cho JY, Kim HS, Kim KL, Jung JS, Huh SO, Suh HW, Kim YH, Song DK (2001) Protection against beta-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br J Pharmacol 133:89–96
Cheng CY, Su SY, Tang NY, Ho TY, Chiang SY, Hsieh CL (2008) Ferulic acid provides neuroprotectionagainst oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res 13:136–150
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Belle NAV, Dalmolin GD, Fonini G, Rubim MA, Rocha JBT (2004) Polyamines reduces lipid peroxidation induced by different pro-oxidant agents. Brain Res 1008:245–251
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free Rad Biol Med 26:1231–1237
Kwon SH, Lee HK, Kim JA, Hong SI, Kim HC, Jo TH, Park YI, Lee CK, Kim YB, Lee SY, Jang CG (2010) Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur J Pharmacol 649:210–217
Katalinic M, Rusak G, Domacinovic Barovic J, Sinko G, Jelic D, Antolovic R, Kovarik Z (2010) Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur J Med Chem 45:186–192
Howes MJR, Perry NSL, Houghton PJ (2003) Plants with traditional uses and activities, relevant to the management of Alzheimer’s disease and other cognitive disorders. Phytother Res 17:1–18
Orhan I, Kartal M, Tosun F, Sener B (2007) Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Z Naturforsch 62c:829–832
Oboh G, Ademiluyi AO, Akinyemi AJ (2012) Inhibition of acetylcholinesterase activities and some pro-oxidant induced lipid peroxidation in rat brain by two varieties of ginger (Zingiber officinale). Exp Toxicol Pathol 64(4):315–319
Martinez GR, Loureiro AP, Marques SA, Miyamoto S, Yamaguchi LF, Onuki J, Almeida EA, Garcia CC, Barbosa LF, Medeiros MH, Di mascio P (2003) Oxidative and alkylating damage in DNA. Mutat Res 544:115–127
Parihar MS, Hemnani T (2004) Alzheimer’s disease pathogenesis and therapeutic interventions. J Clin Neurosci 11:456–467
Stone TW, Perkins MN (1981) Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur J Pharmacol 72:411–412
Guillemin G, Meininger V, Brew B (2006) Implications for the kynurenine pathway and quinolinic acid in amyotrophic lateral sclerosis. Neurodeg Dis 2:166–176
Oboh G, Akinyemi AJ, Ademiluyi AO (2012) Antioxidant and inhibitory effect of red ginger (Zingiber officinale var. Rubra) and white ginger (Zingiber officinale Roscoe) on Fe2+ induced lipid peroxidation in rat brain in vitro. Exp Toxicol Pathol 64:31–36
Cho ES, Jang YJ, Hwang MK, Kang NJ, Lee KW, Lee HJ (2009) Attenuation of oxidative neuronal cell death by coffee phenolic phytochemicals. Mutat Res 661:18–24
Butterfield DA, Castegna A, Pocernich CB, Drake J, Scapagnini G, Calabrese V (2002) Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J Nutr Biochem 13:444–461
Butterfield DA, Lauderback CM (2002) Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid-peptide-associated free radical oxidative stress. Free Radic Biol Med 32:1050–1060
Gumbinger HG, Vahlensieck U, Winterhoff H (1993) Metabolism of caffeic acid in the isolated perfused rat liver. Planta Med 59:491–493
Uang YS, Kang FL, Hsu KY (1995) Determination of caffeic acid in rabbit plasma by high-performance liquid chromatography. J Chromatogr Biomed Sci Appl 673:43–49
Simonetti P, Gardana C, Pietta P (2001) Plasma levels of caffeic acid and antioxidant status after red wine intake. J Agric Food Chem 49:5964–5968
Ito H, Sun XL, Watanabe M, Okamoto M, Hatano T (2008) Chlorogenic acid and its metabolite m-coumaric acid evoke neurite outgrowth in hippocampal neuronal cells. Biosci Biotechnol Biochem 72:885–888
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Oboh, G., Agunloye, O.M., Akinyemi, A.J. et al. Comparative Study on the Inhibitory Effect of Caffeic and Chlorogenic Acids on Key Enzymes Linked to Alzheimer’s Disease and Some Pro-oxidant Induced Oxidative Stress in Rats’ Brain-In Vitro. Neurochem Res 38, 413–419 (2013). https://doi.org/10.1007/s11064-012-0935-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0935-6