Abstract
Hedgehog (Hh) signaling regulates the growth of malignant gliomas by a ligand-dependent mechanism. The cellular source of Sonic Hh ligand and mode of signaling have not been clearly defined due to the lack of methods to definitively identify neoplastic cells in glioma specimens. Using an antibody specific for mutant isocitrate dehydrogenase protein expression to identify glioma cells, we demonstrate that Sonic Hh ligand and the pathway components Patched1 (PTCH1) and GLI1 are expressed in neoplastic cells. Further, Sonic Hh ligand and its transcriptional targets, PTCH1 and GLI1, are expressed in mutually distinct populations of neoplastic cells. These findings support a paracrine mode of intratumoral Hh signaling in malignant gliomas.



Similar content being viewed by others
References
Varjosalo M, Taipale J (2008) Hedgehog: functions and mechanisms. Genes Dev 22:2454–2472
Barakat MT, Humke EW, Scott MP (2010) Learning from Jekyll to control Hyde: Hedgehog signaling in development and cancer. Trends Mol Med 16:337–348
Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, Nannini-Pepe M, Kotkow K, Marsters JC, Rubin LL, de Sauvage FJ (2008) A paracrine requirement for hedgehog signalling in cancer. Nature 455:406–410
Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, Gervaz P, Ruiz i Altaba A (2009) Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med 1:338–351
Berman DM, Karhadkar SS, Maitra A, De Oca Montes R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA (2003) Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature 425:846–851
Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernandez-del Castillo C, Yajnik V, Antoniu B, McMahon M, Warshaw AL, Hebrok M (2003) Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 425:851–856
Park KS, Martelotto LG, Peifer M, Sos ML, Karnezis AN, Mahjoub MR, Bernard K, Conklin JF, Szczepny A, Yuan J, Guo R, Ospina B, Falzon J, Bennett S, Brown TJ, Markovic A, Devereux WL, Ocasio CA, Chen JK, Stearns T, Thomas RK, Dorsch M, Buonamici S, Watkins DN, Peacock CD, Sage J (2011) A crucial requirement for Hedgehog signaling in small cell lung cancer. Nat Med 17:1504–1508
Singh S, Wang Z, Liang Fei D, Black KE, Goetz JA, Tokhunts R, Giambelli C, Rodriguez-Blanco J, Long J, Lee E, Briegel KJ, Bejarano PA, Dmitrovsky E, Capobianco AJ, Robbins DJ (2011) Hedgehog-producing cancer cells respond to and require autocrine Hedgehog activity. Cancer Res 71:4454–4463
Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A (2007) HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol 17:165–172
Ehtesham M, Sarangi A, Valadez JG, Chanthaphaychith S, Becher MW, Abel TW, Thompson RC, Cooper MK (2007) Ligand-dependent activation of the hedgehog pathway in glioma progenitor cells. Oncogene 26:5752–5761
Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, Eberhart CG (2007) Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells 25:2524–2533
Sarangi A, Valadez JG, Rush S, Abel TW, Thompson RC, Cooper MK (2009) Targeted inhibition of the Hedgehog pathway in established malignant glioma xenografts enhances survival. Oncogene 28:3468–3476
Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB (2003) Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature 422:313–317
Merchant AA, Matsui W (2010) Targeting Hedgehog—a cancer stem cell pathway. Clin Cancer Res 16:3130–3140
Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A (2008) Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol (Berl) 116:597–602
Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, Koboldt DC, Fulton RS, Delehaunty KD, McGrath SD, Fulton LA, Locke DP, Magrini VJ, Abbott RM, Vickery TL, Reed JS, Robinson JS, Wylie T, Smith SM, Carmichael L, Eldred JM, Harris CC, Walker J, Peck JB, Du F, Dukes AF, Sanderson GE, Brummett AM, Clark E, McMichael JF, Meyer RJ, Schindler JK, Pohl CS, Wallis JW, Shi X, Lin L, Schmidt H, Tang Y, Haipek C, Wiechert ME, Ivy JV, Kalicki J, Elliott G, Ries RE, Payton JE, Westervelt P, Tomasson MH, Watson MA, Baty J, Heath S, Shannon WD, Nagarajan R, Link DC, Walter MJ, Graubert TA, DiPersio JF, Wilson RK, Ley TJ (2009) Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med 361:1058–1066
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812
Paschka P, Schlenk RF, Gaidzik VI, Habdank M, Kronke J, Bullinger L, Spath D, Kayser S, Zucknick M, Gotze K, Horst HA, Germing U, Dohner H, Dohner K (2010) IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol 28:3636–3643
Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, Marks KM, Prins RM, Ward PS, Yen KE, Liau LM, Rabinowitz JD, Cantley LC, Thompson CB, Vander Heiden MG, Su SM (2009) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462:739–744
Gross S, Cairns RA, Minden MD, Driggers EM, Bittinger MA, Jang HG, Sasaki M, Jin S, Schenkein DP, Su SM, Dang L, Fantin VR, Mak TW (2010) Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med 207:339–344
Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, Ito S, Yang C, Xiao MT, Liu LX, Jiang WQ, Liu J, Zhang JY, Wang B, Frye S, Zhang Y, Xu YH, Lei QY, Guan KL, Zhao SM, Xiong Y (2011) Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 19:17–30
Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, Felsberg J, Wolter M, Mawrin C, Wick W, Weller M, Herold-Mende C, Unterberg A, Jeuken JW, Wesseling P, Reifenberger G, von Deimling A (2009) Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol (Berl) 118:469–474
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773
Watanabe T, Nobusawa S, Kleihues P, Ohgaki H (2009) IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol 174:1149–1153
Bettegowda C, Agrawal N, Jiao Y, Sausen M, Wood LD, Hruban RH, Rodriguez FJ, Cahill DP, McLendon R, Riggins G, Velculescu VE, Oba-Shinjo SM, Marie SK, Vogelstein B, Bigner D, Yan H, Papadopoulos N, Kinzler KW (2011) Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science 333:1453–1455
Jiao Y, Killela PJ, Reitman ZJ, Rasheed AB, Heaphy CM, de Wilde RF, Rodriguez FJ, Rosemberg S, Oba-Shinjo SM, Nagahashi Marie SK, Bettegowda C, Agrawal N, Lipp E, Pirozzi C, Lopez G, He Y, Friedman H, Friedman AH, Riggins GJ, Burger P, McLendon R, Bigner DD, Vogelstein B, Meeker AK, Kinzler KW, Papadopoulos N, Diaz LA, Yan H (2012) Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget 3:709–722
Liu XY, Gerges N, Korshunov A, Sabha N, Khuong-Quang DA, Fontebasso AM, Fleming A, Hadjadj D, Schwartzentruber J, Majewski J, Dong Z, Siegel P, Albrecht S, Croul S, Jones DT, Kool M, Tonjes M, Reifenberger G, Faury D, Zadeh G, Pfister S, Jabado N (2012) Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol (Berl) 124:615–625
Hartmann C, Hentschel B, Tatagiba M, Schramm J, Schnell O, Seidel C, Stein R, Reifenberger G, Pietsch T, von Deimling A, Loeffler M, Weller M (2011) Molecular markers in low-grade gliomas: predictive or prognostic? Clin Cancer Res 17:4588–4599
Weller M, Felsberg J, Hartmann C, Berger H, Steinbach JP, Schramm J, Westphal M, Schackert G, Simon M, Tonn JC, Heese O, Krex D, Nikkhah G, Pietsch T, Wiestler O, Reifenberger G, von Deimling A, Loeffler M (2009) Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol 27:5743–5750
Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, Fink K, Souhami L, Laperriere N, Curran W, Mehta M (2013) Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol 31:337–343
Capper D, Weissert S, Balss J, Habel A, Meyer J, Jager D, Ackermann U, Tessmer C, Korshunov A, Zentgraf H, Hartmann C, von Deimling A (2010) Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol 20:245–254
van den Bent MJ, Dubbink HJ, Marie Y, Brandes AA, Taphoorn MJ, Wesseling P, Frenay M, Tijssen CC, Lacombe D, Idbaih A, van Marion R, Kros JM, Dinjens WN, Gorlia T, Sanson M (2010) IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin Cancer Res 16:1597–1604
Gerardo Valadez J, Grover VK, Carter MD, Calcutt MW, Abiria SA, Lundberg CJ, Williams TV, Cooper MK (2013) Identification of Hedgehog pathway responsive glioblastomas by isocitrate dehydrogenase mutation. Cancer Lett 328:297–306
Becher OJ, Hambardzumyan D, Fomchenko EI, Momota H, Mainwaring L, Bleau AM, Katz AM, Edgar M, Kenney AM, Cordon-Cardo C, Blasberg RG, Holland EC (2008) Gli activity correlates with tumor grade in platelet-derived growth factor-induced gliomas. Cancer Res 68:2241–2249
Paulus W, Peiffer J (1989) Intratumoral histologic heterogeneity of gliomas. A quantitative study. Cancer 64:442–447
Snuderl M, Fazlollahi L, Le LP, Nitta M, Zhelyazkova BH, Davidson CJ, Akhavanfard S, Cahill DP, Aldape KD, Betensky RA, Louis DN, Iafrate AJ (2011) Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell 20:810–817
Sottoriva A, Spiteri I, Piccirillo SG, Touloumis A, Collins VP, Marioni JC, Curtis C, Watts C, Tavare S (2013) Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci USA 110:4009–4014
Szerlip NJ, Pedraza A, Chakravarty D, Azim M, McGuire J, Fang Y, Ozawa T, Holland EC, Huse JT, Jhanwar S, Leversha MA, Mikkelsen T, Brennan CW (2012) Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proc Natl Acad Sci USA 109:3041–3046
Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366:883–892
Swanton C (2012) Intratumor heterogeneity: evolution through space and time. Cancer Res 72:4875–4882
Ghali L, Wong ST, Green J, Tidman N, Quinn AG (1999) Gli1 protein is expressed in basal cell carcinomas, outer root sheath keratinocytes and a subpopulation of mesenchymal cells in normal human skin. J Invest Dermatol 113:595–599
Grover VK, Valadez JG, Bowman AB, Cooper MK (2011) Lipid modifications of Sonic hedgehog ligand dictate cellular reception and signal response. PLoS ONE 6:e21353
Ruiz i Altaba A (2008) Therapeutic inhibition of Hedgehog-GLI signaling in cancer: epithelial, stromal, or stem cell targets? Cancer Cell 14:281–283
Guha M (2012) Hedgehog inhibitor gets landmark skin cancer approval, but questions remain for wider potential. Nat Rev Drug Discov 11:257–258
Theunissen JW, de Sauvage FJ (2009) Paracrine Hedgehog signaling in cancer. Cancer Res 69:6007–6010
McMillan R, Matsui W (2012) Molecular pathways: the hedgehog signaling pathway in cancer. Clin Cancer Res 18:4883–4888
Piaskowski S, Bienkowski M, Stoczynska-Fidelus E, Stawski R, Sieruta M, Szybka M, Papierz W, Wolanczyk M, Jaskolski DJ, Liberski PP, Rieske P (2011) Glioma cells showing IDH1 mutation cannot be propagated in standard cell culture conditions. Br J Cancer 104:968–970
Luchman HA, Stechishin OD, Dang NH, Blough MD, Chesnelong C, Kelly JJ, Nguyen SA, Chan JA, Weljie AM, Cairncross JG, Weiss S (2012) An in vivo patient-derived model of endogenous IDH1-mutant glioma. Neuro Oncol 14:184–191
Acknowledgments
This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development through grant 1 I01 BX000744-01. The contents do not represent the views of the Department of Veterans Affairs or the United States Government. We are particularly indebted to patients at Vanderbilt University Medical Center who provided invaluable research material for the Molecular Neurosurgical Tissue Bank. We thank those who established and maintain the Tissue Bank, Reid C. Thompson MD (principal investigator), Cherryl Kinnard RN (research nurse) and Larry A. Pierce MS (manager). Histological services were performed, in part, by the Vanderbilt University Medical Center (VUMC) Translational Pathology Shared Resource (supported by award 5P30 CA068485 to the Vanderbilt-Ingram Cancer Center). We thank Li-Chong Wang PhD and Ruby Hsu PhD at Advanced Cell Diagnostics for expertise and assistance with in situ hybridization studies.
Conflict of interest
The authors of this study declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sunday A. Abiria, Thomas V. Williams and Alexander L. Munden have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1
Comparable IDH1 R132H staining with chromogenic and fluorescence detection. Paraffin sections of an IDH1 R132H mutant anaplastic oligodendroglioma (12262 AO) and a wild type glioblastoma (15589 GBM) were stained with anti-IDH1 R132H antibody and then processed for detection by immunohistochemistry (A and B) or immunofluorescence (C and D) (EPS 3206 kb)
Fig. 2
Specificity of SHH staining. An astrocytoma (13396 A) was stained with anti-Shh antibody (A) or with secondary antibody alone (B). In other control experiments, anti-Shh antibody was preincubated with a Shh blocking peptide (C) or with a control blocking peptide (D). Immunostaining was detected using Alexa Fluor 555 (red) and nuclei were counterstained with Hoechst dye (blue) (EPS 2774 kb)
Fig. 3
SHH expression in IDH1 R132H-mutant glioma cells. Paraffin sections of IDH1 mutant gliomas were immunostained for IDH1 R132H (green) and SHH (red) and nuclei were counterstained with Hoechst dye (blue). (A) SHH expression was detected in IDH1 R132H-positive cells in 11 of 12 glioma specimens, and the percentage of double-positive cells in each high-powered field (points on the graph) varied within and among each specimen. (B-I) Colocalization of SHH and IDH1 R132H immunofluorescence staining in a cytosolic expression pattern in an anaplastic oligodendroglioma (12262 AO) (B-E and I), oligodendroglioma (12784 O), astrocytoma (13396 A), and oligodendroglioma (16772 O) (EPS 3258 kb)
Fig. 4
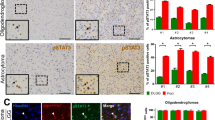
Validation of two commercially available anti-Gli1 antibodies. Epilepsy and glioma specimens were stained for GLI1 using a goat anti-Gli1 antibody (C-18, sc-6152) from Santa Cruz Biotechnology (A and B), or a mouse anti-Gli1 antibody (2643S) from Cell Signaling Technologies and then for SHH (C and D). Inset demonstrates SHH (brown) and GLI1 (purple) staining in separate cells in a glioma specimen (EPS 10122 kb)
Fig. 5
Validation of chromogenic multiplex in situ hybridization for SHH and GLI1. Glioma paraffin sections were evaluated for background signal following hybridization with probes for the bacterial gene DapB transcript (A) and for expression of transcripts from housekeeping human genes POLR2A and PPIB (B). Compared to the negative (A) and positive (B) controls, GLI1 (green) and SHH (red) transcripts were detected at moderate levels (C) (EPS 6836 kb)
Rights and permissions
About this article
Cite this article
Abiria, S.A., Williams, T.V., Munden, A.L. et al. Expression of Hedgehog ligand and signal transduction components in mutually distinct isocitrate dehydrogenase mutant glioma cells supports a role for paracrine signaling. J Neurooncol 119, 243–251 (2014). https://doi.org/10.1007/s11060-014-1481-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1481-7