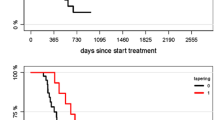
Abstract
Bevacizumab ((BEV) has become a mainstay of treating recurrent glioblastoma, but eventual tumor resistance is expected. Targeting multiple growth-associated signaling pathways may result in more effective treatment than targeting VEGF alone. Patients with recurrent glioblastoma were stratified by prior BEV exposure and treated with sunitinib 37.5 mg daily in this phase II study. Response evaluations were performed at baseline and at the end of every 4 week cycle. Six-month progression-free survival (PFS6) was the primary endpoint for both arms of the study. Secondary endpoints included health related quality of life measures and FDG-PET correlatives with patient outcomes. Sixty-three patients were accrued to this study; thirty-two were BEV-naïve, 31 were BEV-resistant. PFS6 was 10.4 % [95 % CI 3.2–33.8] in the BEV-naïve cohort and 0 % in the BEV-resistant cohort. Median overall survival was 9.4 months [95 % CI 6.15–21.90] in the BEV-naïve cohort and 4.37 months [95 % CI 3.02–6.21] in the BEV-resistant cohort. 3/29 patients (10 %) of the BEV-naïve, and 0/27 BEV-resistant patients achieved radiographic response. Thrombocytopenia, leukopenia, and neutropenia were the most common drug-associated adverse events and occurred with higher frequency than expected. Sunitinib treatment in BEV-naïve patients did not appear to affect outcomes with subsequent BEV therapy. Continuous daily sunitinib did not prolong progression-free survival in BEV-naïve nor BEV-resistant patients with recurrent glioblastoma.
Similar content being viewed by others
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996. doi:10.1056/NEJMoa043330
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27(5):740–745. doi:10.1200/JCO.2008.16.3055
Iwamoto FM, Abrey LE, Beal K, Gutin PH, Rosenblum MK, Reuter VE, DeAngelis LM, Lassman AB (2009) Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology 73(15):1200–1206. doi:10.1212/WNL.0b013e3181bc0184
Norden AD, Drappatz J, Wen PY (2008) Novel anti-angiogenic therapies for malignant gliomas. Lancet Neurol 7(12):1152–1160. doi:10.1016/S1474-4422(08)70260-6
Chow LQ, Eckhardt SG (2007) Sunitinib: from rational design to clinical efficacy. J Clin Oncol 25(7):884–896. doi:10.1200/JCO.2006.06.3602
de Bouard S, Herlin P, Christensen JG, Lemoisson E, Gauduchon P, Raymond E, Guillamo JS (2007) Antiangiogenic and anti-invasive effects of sunitinib on experimental human glioblastoma. Neuro Oncol 9(4):412–423. doi:10.1215/15228517-2007-024
Neyns B, Sadones J, Chaskis C, Dujardin M, Everaert H, Lv S, Duerinck J, Tynninen O, Nupponen N, Michotte A, De Greve J (2011) Phase II study of sunitinib malate in patients with recurrent high-grade glioma. J Neurooncol 103(3):491–501. doi:10.1007/s11060-010-0402-7
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28(11):1963–1972. doi:10.1200/JCO.2009.26.3541
Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA (1995) The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer 75(5):1151–1161
Kreisl TN, Zhang W, Odia Y, Shih JH, Butman JA, Hammoud D, Iwamoto FM, Sul J, Fine HA (2011) A phase II trial of single-agent bevacizumab in patients with recurrent anaplastic glioma. Neuro Oncol 13(10):1143–1150. doi:10.1093/neuonc/nor091
Ballman KV, Buckner JC, Brown PD, Giannini C, Flynn PJ, LaPlant BR, Jaeckle KA (2007) The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol 9(1):29–38. doi:10.1215/15228517-2006-025
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740. doi:10.1200/JCO.2008.19.8721
Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ (2010) Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol 66(2):357–371. doi:10.1007/s00280-009-1170-y
Barrios CH, Hernandez-Barajas D, Brown MP, Lee SH, Fein L, Liu JH, Hariharan S, Martell BA, Yuan J, Bello A, Wang Z, Mundayat R, Rha SY (2011) Phase II trial of continuous once-daily dosing of sunitinib as first-line treatment in patients with metastatic renal cell carcinoma. Cancer. doi:10.1002/cncr.26440
Goldlust SA, Cavaliere R, Newton HB, Hsu M, Deangelis LM, Batchelor TT, Gilbert MR, Lassman AB (2011) Bevacizumab for glioblastoma refractory to vascular endothelial growth factor receptor inhibitors. J Neurooncol. doi:10.1007/s11060-011-0768-1
Acknowledgments
Authors would like to thank the Neuro-Oncology Branch clinical research staff for their tireless commitment to this project: Maria Gonzalez, Laurie Rosenblatt, Charisse Garcia, Tracy Cropper, Julie Perreti, Cheryl Royce, Nancy Garren, Irene Haggarty, Megan Mackey, Leslie Moses, Colleen Livingstone, Yazmin Odia, Katharine McNeill. We also thank the patients that volunteered to participate in the study whom make this work possible.
Conflict of interest
No authors have any conflicts to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kreisl, T.N., Smith, P., Sul, J. et al. Continuous daily sunitinib for recurrent glioblastoma. J Neurooncol 111, 41–48 (2013). https://doi.org/10.1007/s11060-012-0988-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-012-0988-z