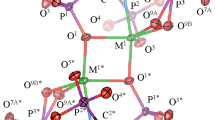
Abstract
The results of first principles calculations of band structure, density of states and electron density topology of ZnC2O4 crystal are presented. The calculations have been performed with WIEN2k FP LAPW ab initio package. The obtained SCF electron density has been used in calculations of Bader’s QTAIM (quantum theory of atoms in molecules) topological properties of the electron density in crystal. Additional calculations of bond orders (Pauling, Bader, Cioslowski and Mixon) and bond valences according to bond valence model have been done. The obtained results are analyzed from the point of view of the thermal decomposition process, and this analysis indicates, that most probably this compound should decompose to metal oxide, carbon oxide and carbon dioxide, in agreement with the experiment.
Similar content being viewed by others
References
Y. D. Kondrashev, V. S. Bogdanov, S. N. Golubev and G. F. Pron, Zh. Struct. Khim., 26 (1985) 90.
E. Jeanneau, N. Audebrand and D. Louer, Acta Cryst., C57 (2001) 1012.
B. Małecka, E. Drożdż-Cieśla and A. Małecki, Thermochim. Acta, 423 (2004) 13.
M. E. Brown, D. Dollimore and A. K. Galwey, Comprehensive Chemical Kinetics, Vol. 22. Reactions In Solid State, C. H. Bamford and C. F. H. Tipper, Eds, Elsevier Amsterdam 1980.
V. V. Boldyrev, I. S. Nevyantsev, Y. I. Mikhailov and E. F. Khayretdinov, Kinet. Katal., 11 (1970) 367.
H. J. Borchardt and F. Daniels, J. Am. Chem. Soc., 79 (1957) 41.
D. Dollimore, Thermochim. Acta, 117 (1987) 331.
B. S. Randhawa and M. Kaur, J. Therm. Anal. Cal., 89 (2007) 251.
A. K. Galwey and M.E. Brown, J. Therm. Anal. Cal., 90 (2007) 9.
J. Fujita, K. Nakamoto and M. Kobayashi, J. Phys. Chem., 61 (1957) 1014.
S. Rane, H. Uskaikar, R. Pednekar and R. Mhalsikar, J. Therm. Anal. Cal., 90 (2007) 627.
P. Blaha, K. Schwarz, G. K. H. Madsen, D. Kvasnicka and J. Luitz, WIEN2k, An Augmented Plane Wave+Local Orbitals Program for Calculating Crystal Properties (Karlheinz Schwarz, Techn. Universität Wien, Austria), ISBN 3-9501031-1-2, (2001).
J. C. Slater, Phys. Rev., 51 (1937) 151.
T. L. Loucks, Augmented Plane Wave Method, Benjamin, New York 1967.
O. K. Andersen, Solid State Commun., 13 (1973) 133.
D. R. Hamann, Phys. Rev. Lett., 42 (1979) 662.
E. Wimmer, H. Krakauer, M. Weinert and A. J. Freeman, Phys. Rev., B24 (1981) 864.
D. J. Singh, Planewaves, Pseudopotentials and the LAPW Method, Kluwer Academic Publishers, Dordrecht 1994.
A. Koleżyski and A. Małecki, J. Therm. Anal. Cal., sent to editor.
J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 77 (1996) 3865.
R. F. W. Bader, Atoms in Molecules: A Quantum Theory, Clarendon Press, Oxford 1990.
L. Pauling, The Nature of the Chemical Bond, Cornell University Press, Ithaca, New York 1960.
R. F. W. Bader, T. S. Slee, D. Cremer and E. Kraka, J. Am. Chem. Soc., 105 (1983) 5061.
J. Cioslowski and S. T. Mixon, J. Am. Chem. Soc., 113 (1991) 4142.
J. L. Jules and J. R. Lombardi, J. Mol. Struct. (Teochem.), 664–665 (2003) 255.
I. D. Brown, The Chemical Bond in Inorganic Chemistry. The Bond Valence Model., Oxford University Press, 2002.
A. Koleżyski and A. Małecki, J. Therm. Anal. Cal., sent to editor.
S. T. Howard and O. Lamarche, J. Phys. Org. Chem., 16 (2003) 133.
J. D. Danforth and J. Dix, J. Am. Chem. Soc., 93 (1971) 6843.
Z. Gabelica, R. Hubin and E. G. Derouane, Thermochim. Acta, 24 (1978) 315.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Koleżyński, A., Małecki, A. First principles studies of thermal decomposition of anhydrous zinc oxalate. J Therm Anal Calorim 96, 645–651 (2009). https://doi.org/10.1007/s10973-008-9494-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-008-9494-0