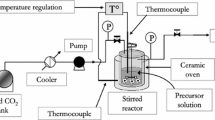
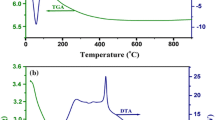
Abstract
Stabilised titania sols were prepared using an additive free particulate sol-gel route, via electrostatic stabilisation mechanism, with various processing parameters. Peptisation temperature, 50°C and 70°C, and TiO2 concentration, 0.1, 0.2 and 0.4 molar, were chosen as processing parameters during sol preparation. Results from TiO2 particle size and zeta potential of sols revealed that the smallest titania hydrodynamic diameter (13 nm) and the highest zeta potential (47.7 mV) were obtained for the sol produced at the lower peptisation temperature of 50°C and lower TiO2 concentration of 0.1 M. On the other hand, between the sols prepared at 70°C, smaller titania particles (20 nm) and higher zeta potential (46.3 mV) were achieved with increasing TiO2 concentration up to 0.4 M. X-ray diffraction (XRD) and Brunauer-Emmett-Teller (BET) results of produced powders annealed at different temperatures showed that the 300°C annealed powder made from 0.1 M sol prepared at 50°C was a mixture of anatase and brookite, corresponding to a major phase of anatase (∼95% estimated), with the smallest average crystallite size of 1.3 nm and the highest specific surface area (SSA) of 193 m2/g. Furthermore, increasing TiO2 concentration up to 0.4 molar for the sols prepared at 70°C resulted in decreasing the average crystallite size (1.9 nm at 300°C) and increasing SSA (116 m2/g at 300°C) of the powders annealed at different temperatures. Anatase-to-rutile phase transformation temperature was increased with decreasing peptisation temperature down to 50°C, whereas TiO2 concentration had no effect on this transition. Anatase percentage increased with decreasing both peptisation temperature and TiO2 concentration. Such prepared powders can be used in many applications in areas from photo catalysts to gas sensors.
Similar content being viewed by others
References
Bonini N, Carotta MC, Chiorini A, Guidi V, Malagu C, Martinelli G, Paglialonga L, Sacerdoti M (2000) Sensors Actuators B 68:274
Perera VPS, Jayaweera PVV, Pitigala PKDDP, Andaranayake PKMB, Hastings G, Perera AGU, Tennakone K (2004) Synth Met 143:283
Mao D, Lu G, Chen Q (2004) Applied Catalysis A: General 263:83
Huang Y, Kavan L, Exnar I, Gratzel M (1995) J Electrochem. Society 142:L142
Aliev AE, Shin HW (2002) Displays 23:239
Fretwell R, Douglas P, (2001) Photochem J Photobiol A: Chem 143:229
Tai WP, Oh JH (2002) Sensors and Actuators B 85:154
Francioso L, Presicce DS, Taurino AM, Rella R, Siciliano P, Ficarella A (2003) Sensors Actuators B 95:66
Inagaki M, Nakazawa Y, Hirano M, Kobayashi Y, Toyoda M (2001) J Inorg Mater 3:809
Tanner RE, Liang Y, Altman EI (2002) Surface Science 506:251
Shimizu K, Imai H, Hirashima H, Tsukuma K (1999) Thin Solid Films 351:220
Blesic MD, Saponjic ZV, Nedeljkovic JM, Uskokovic DP (2002) Mater Lett 54:298
Carotta MC, Ferroni M, Gnani D, Guidi V, Merli M, Martinelli G, Casale MC, Notaro M (1999) Sensors Actuators B 58:310
Lee DS, Han SD, Huh JS, Lee DD (1999) Sensors Actuators B 60:57
Ruiz AM, Arbiol J, Cornet A, Shimanoe K, Morante JR, Yamazoe N (2005) Mater Res Soc 828:A4.10.1
Liu X, Yang J, Wang L, Yang X, Lu L, Wang X (2000) Mater Sci Engi A 289:241
Devi GS, Hyodo T, Shimizu Y, Egashira M (2002) Sensors Actuators B 87:122
Garzella C, Comini E, Tempesti E, Frigeri C, Sberveglieri G (2000) Sensors Actuators B 68:189
Keshmiri M, Mohseni M, Troczynski T (2004) Applied Catalysis B: Environmental 53:209
Chen W, Zhang J, Fang Q, Li S, Wu J, Li F, Jiang K (2004) Sensors Actuators B 100:195
Miki T, Nishizawa K, Suzuki K, Kato K (2004) Mater Lett 58:2751
Chemseddine A, Moritz T (1999) Eur J Inorg Chem 235
Zhang H, Finnegan M, Banfield JF (2001) Nano Letters 1:81
Sivakumar S, Krishna Pillai P, Mukundan P, Warrier KGK (2002) Mater Lett 57:330
Pottier A, Cassaignon S, Chaneac C, Villain F, Tronc E, Jolivet JP (2003) J Mater Chem 13:877
Cordero-Cabrera MC, Walker GS, Grant D (2005) J Mater Sci 40:3709
Spurr R, Myers H (1957) Anal Chem 29:760
Cullity BD (1978) Elements of X-ray diffraction, Addison-Wesley Publishing Company Inc, London, p 99
Malvern instruments (2003) DST customer training manual for zeta potential, chapter 6
Socrates G (1994) Infrared characteristic group frequencies: Tables and charts, Second Edition John Wiley & Sons, England, p 62/237
Ivanova T, Harizanova A, Surtchev M (2002) Mater Lett 55:327
Carp O, Huisman CL, Reller A (2004) Progress in Solid State Chemistry 32:33
Diebold U (2003) Surface Science Reports 48:53
Bokhimi X, Morales A, Pedraza F (2002) J Solid State Chem 169:176
JCPDS PDF-2pattern 73–1764
Mohammadi MR, Cordero-Cabrera MC, Fray DJ, Ghorbani M, (2006) In Press in Journal of Sensors Actuators B
Mohammadi MR, Ghorbani M, Fray DJ (2006) In Press in J Mater Sci Techn
Mohammadi MR, Ghorbani M, Cordero-Cabrera MC, Fray DJ (2006) In Press in J Mater Sci
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammadi, M.R., Cordero-Cabrera, M.C., Ghorbani, M. et al. Synthesis of high surface area nanocrystalline anatase-TiO2 powders derived from particulate sol-gel route by tailoring processing parameters. J Sol-Gel Sci Technol 40, 15–23 (2006). https://doi.org/10.1007/s10971-006-8267-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-006-8267-0