Abstract
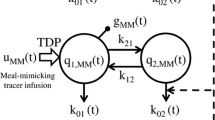
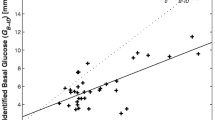
The integrated glucose–insulin (IGI) model is a previously developed semi-mechanistic model that incorporates control mechanisms for the regulation of glucose production, insulin secretion, and glucose uptake. It has been shown to adequately describe insulin and glucose profiles in both type 2 diabetics and healthy volunteers following various glucose tolerance tests. The aim of this study was to investigate the ability of the IGI model to correctly identify the primary mechanism of action of glibenclamide (Gb), based on meal tolerance test (MTT) data in healthy volunteers. IGI models with different mechanism of drug action were applied to data from eight healthy volunteers participating in a randomized crossover study with five single-dose tests (placebo and four drug arms). The study participants were given 3.5 mg of Gb, intravenously or orally, or 3.5 mg of the two main metabolites M1 and M2 intravenously, 0.5 h prior to a standardized breakfast with energy content of 1800 kJ. Simultaneous analysis of all data by nonlinear mixed effect modeling was performed using NONMEM®. Drug effects that increased insulin secretion resulted in the best model fit, thus identifying the primary mechanism of action of Gb and metabolites as insulin secretagogues. The model also quantified the combined effect of Gb, M1 and M2 to have a fourfold maximal increase on endogenous insulin secretion, with an EC50 of 169.1 ng mL−1 for Gb, 151.4 ng mL−1 for M1 and 267.1 ng mL−1 for M2. The semi-mechanistic IGI model was successfully applied to MTT data and identified the primary mechanism of action for Gb, quantifying its effects on glucose and insulin time profiles.




Similar content being viewed by others
References
Tornøe CW, Agersø H, Senderovitz T, Nielsen HA, Madsen H, Karlsson MO, Jonsson EN (2007) Population pharmacokinetic/pharmacodynamic (PK/PD) modelling of the hypothalamic-pituitary-gonadal axis following treatment with GnRH analogues. Br J Clin Pharmacol 63(6):648–664
Friberg LE, Karlsson MO (2003) Mechanistic models for myelosuppression. Investig New Drugs 21(2):183–194
Nielsen EI, Viberg A, Löwdin E, Cars O, Karlsson MO, Sandström M (2007) Semimechanistic pharmacokinetic/pharmacodynamic model for assessment of activity of antibacterial agents from time-kill curve experiments. Antimicrob Agents Chemother 51(1):128–136
Silber HE, Jauslin PM, Frey N, Karlsson MO (2010) An integrated model for the glucose-insulin system. Basic Clin Pharmacol Toxicol 106(3):189–194
Bergman RN, Ider YZ, Bowden CR, Cobelli C (1979) Quantitative estimation of insulin sensitivity. Am J Physiol 236:E667–E677
De Gaetano A, Arino O (2000) Mathematical modelling of the intravenous glucose tolerance test. J Math Biol 40(2):136–168
Jauslin PM, Karlsson MO, Frey N (2011) Identification of the mechanism of action of a glucokinase activator from oral glucose tolerance test data in type 2 diabetic patients based on an integrated glucose-insulin model. J Clin Pharmacol. [Epub ahead of print] doi: 10.1177/0091270011422231. Accessed 15 October 2012
Jauslin PM, Silber HE, Frey N, Gieschke R, Simonsson USH, Jorga K, Karlsson MO (2007) An integrated glucose-insulin model to describe oral glucose tolerance test data in type 2 diabetics. J Clin Pharmacol 47(10):1244–1255
Silber HE, Frey N, Karlsson MO (2010) An integrated glucose-insulin model to describe oral glucose tolerance test data in healthy volunteers. J Clin Pharmacol 50(3):246–256
Silber HE, Jauslin PM, Frey N, Gieschke R, Simonsson USH, Karlsson MO (2007) An integrated model for glucose and insulin regulation in healthy volunteers and type 2 diabetic patients following intravenous glucose provocations. J Clin Pharmacol 47(9):1159–1171
Drucker DJ, Nauck MA (2006) The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368(9548):1696–1705
Peart GF, Boutagy J, Shenfield GM (1989) The metabolism of glyburide in subjects of known debrisoquin phenotype. Clin Pharmacol Ther 45(3):277–284
Fuccella LM, Tamassia V, Valzelli G (1973) Metabolism and kinetics of the hypoglycemic agent glipizide in man–comparison with glibenclamide. J Clin Pharmacol New Drugs 13(2):68–75
Rydberg T, Wahlin-Boll E, Melander A (1991) Determination of glibenclamide and its two major metabolites in human serum and urine by column liquid chromatography. J Chromatogr 564(1):223–233
Rydberg T, Jönsson A, Røder M, Melander A (1994) Hypoglycemic activity of glyburide (glibenclamide) metabolites in humans. Diabetes Care 17(9):1026–1030
Rydberg T, Jonsson A, Melander A (1995) Comparison of the kinetics of glyburide and its active metabolites in humans. J Clin Pharm Ther 20(5):283–295
Clark CA, Gardiner J, McBurney MI, Anderson S, Weatherspoon LJ, Henry DN, Hord NG (2006) Effects of breakfast meal composition on second meal metabolic responses in adults with type 2 diabetes mellitus. Eur J Clin Nutr 60(9):1122–1129
Rydberg T, Jönsson A, Karlsson MO, Melander A (1997) Concentration-effect relations of glibenclamide and its active metabolites in man: modelling of pharmacokinetics and pharmacodynamics. Br J Clin Pharmacol 43(4):373–381
Serrano-Martín X, Payares G, Mendoza-León A (2006) Glibenclamide, a blocker of K + ATP channels, shows antileishmanial activity in experimental murine cutaneous leishmaniasis. Antimicrob Agents Chemother 50(12):4214–4216
Inagaki N, Gonoi T, Clement IvJP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S (1996) A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K + channels. Neuron 16(5):1011–1017
Ariëns EJ, van Rossum JM, Simonis AM (1957) Affinity, intrinsic activity and drug interactions. Pharmacol Rev 9(2):218–236
Beal S, Sheiner LB, Boeckmann A, Bauer RJ (1989–2009) NONMEM user’s guides. Icon Development Solutions, Ellicott City
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO (2011) Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13(2):143–151
Liu Y, Oiki S, Tsumura T, Shimizu T, Okada Y (1998) Glibenclamide blocks volume-sensitive Cl-channels by dual mechanisms. Am J Physiol 275(2 Pt 1):C343–C351
Ripoll C, Lederer WJ, Nichols CG (1993) On the mechanism of inhibition of K(ATP) channels by glibenclamide in rat ventricular myocytes. J Cardiovasc Electrophysiol 4(1):38–47
Schirra J, Katschinski M, Weidmann C, Schäfer T, Wank U, Arnold R, Göke B (1996) Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest 97(1):92–103
Collins PJ, Horowitz M, Cook DJ (1983) Gastric emptying in normal subjects—a reproducible technique using a single scintillation camera and computer system. Gut 24(12):1117–1125
Creutzfeldt W (1979) The incretin concept today. Diabetologia 16(2):75–85
Holst JJ (2003) Implementation of GLP-1 based therapy of type 2 diabetes mellitus using DPP-IV inhibitors. Adv Exp Med Biol 524:263–279
Nauck MA, Siemsglüss J, Ørskov C, Holst JJ (1996) Release of glucagon-like peptide 1 (GLP-1 [7-36 amide]), gastric inhibitory polypeptide (GIP) and insulin in response to oral glucose after upper and lower intestinal resections. Z Gastroenterol 34(3):159–166
Vollmer K, Hoist JJ, Bailer B, Ellrlchmann M, Nauck MA, Schmidt WE, Meier JJ (2008) Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 57(3):678–687
Yamane S, Harada N, Hamasaki A, Muraoka A, Joo E, Suzuki K, Nasteska D, Tanaka D, Ogura M, Harashima SI, Inagaki N (2012) Effects of glucose and meal ingestion on incretin secretion in Japanese subjects with normal glucose tolerance. J Diabetes Invest 3(1):80–85
Pipeleers D, Kiekens R, Ling Z, Wilikens A, Schuit F (1994) Physiologic relevance of heterogeneity in the pancreatic beta-cell population. Diabetologia 37(SUPPL. 2):S57–S64
Acknowledgments
The authors would like to thank Vincent Madelain from the University of Poitiers in France for contributing to the individual PK parameter predictions used in this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choy, S., Hénin, E., van der Walt, JS. et al. Identification of the primary mechanism of action of an insulin secretagogue from meal test data in healthy volunteers based on an integrated glucose-insulin model. J Pharmacokinet Pharmacodyn 40, 1–10 (2013). https://doi.org/10.1007/s10928-012-9281-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-012-9281-1