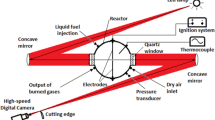
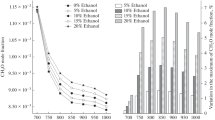
Abstract
Bioethanol is a fuel additive or a fuel substitute that has the benefit of being cleaner and price competitive with gasoline. Therefore, we develop a reduced kinetic mechanism capable of modeling the ethanol combustion and the generation of the combustion products \(\text{H}_{2}\text{O},~\text{CO}_{2},~\text{CO},~ \text{H}_{2},~ \text{C}_{2}\text{H}_{4}\) and OH. Based on a mechanism composed by 372 reversible elementary reactions among 56 reactive species, we propose a reduction strategy to obtain an eight-step mechanism for the ethanol. The reduction strategy consists in estimating the order of magnitude of the reaction rate coefficients, defining the main chain, applying the steady-state and partial equilibrium hypotheses, and justifying the assumptions through an asymptotic analysis. The main advantage of the obtained reduced mechanism is the decrease of the work needed to solve the system of chemical equations proportionally to the number of elementary reactions present in the complete mechanism. Numerical tests are carried out for a jet diffusion flame of ethanol and the results compare well with available data in the literature.




Similar content being viewed by others
References
A.A. Kiss, Comput. Chem. Eng. 34, 812–820 (2010)
P. Bergeron, in Handbook on Bioethanol: Production and Utilization, ed. by C.E. Wyman (Taylor & Francis, New York, 1996), pp. 89–104
K.M. Leung, R.P. Lindstedt, Combust. Flame 102, 129–160 (1995)
H.J. Curran, Proceedings of the European Combust, Meeting (2009)
P.A. Libby, F.A. Williams, Ann. Rev. Fluid Mech. 8, 351–376 (1976)
T. Lu, C.K. Law, Combust. Flame 144, 24–36 (2006)
N. Peters, B. Roog, Reduced Kinetic Mechanisms for Applications in Combustion Systems, Lecture Notes in Physics (Springer, Berlin, 1993)
C.K. Westbrook, Y. Mizobuchi, T.J. Poinsot, P.J. Smith, J. Warnatz, Proc. Combust. Inst. 30, 125–157 (2005)
P. Saxena, F.A. Williams, Proc. Combust. Inst. 31, 1149–1156 (2007)
J. Warnatz, Pure Appl. Chem. 72, 2101–2110 (2000)
H. Bongers, J.A. Van Oijen, L.P.H. De Goey, Proc. Combust. Inst. 29, 1371–1378 (2002)
R.P. Lindstedt, M.P. Meyer, Proc. Combust. Inst. 29, 1395–1402 (2002)
S. Yalamanchili, W.A. Sirignano, R. Seiser, K. Seshadri, Combust. Flame 142, 258–265 (2005)
J. Li, A. Kazakov, M. Chaos, F.L. Dryer, 5th US Combustion Meeting (2007)
O. Röhl, N. Peters, Proceedings of the European Combust, Meeting (2009)
N.P. Komninos, C.D. Rakopoulos, Open Renew. Energ. J. 4, 47–59 (2011)
N.M. Marinov, Int. J. Chem. Kinet. 31, 183–220 (1999)
J. Li, A. Kazakov, F.L. Dryer, J. Phys. Chem. A 108, 7671–7680 (2004)
R. Seiser, S. Humer, K. Seshadri, E. Pucher, Proc. Combust. Inst. 31, 1173–1180 (2007)
S.R. Turns, An Introduction to Combustion: Concepts and Applications, 2nd edn. (McGraw-Hill, Singapore, 2000), pp. 148–177
N. Peters, in Dynamics of Reactive Systems, Part I: Flames. Systematic reduction of flame kinetics: principles and details (Progress in Astronautics and Aeronautics, American Institute of Astronautics and Aeronautics, Monmouth Junction, 1988), pp. 67–86
N.J. Glassmaker, Intrinsic low-dimensional manifold method for rational simplification of chemical kinetics. (1999) http://www.nd.edu/~powers/nick.glassmaker.pdf. Accessed on 13 July 2010.
H. Pitsch, Combust. Flame 123, 358–374 (2000)
H. Pitsch, H. Steiner, Phys. Fluids 12, 2541–2554 (2000)
M.R.H. Sheikhi, T.G. Drozda, P. Givi, F.A. Jaberi, S.B. Pope, Proc. Combust. Inst. 30, 549–556 (2005)
K.K.J. Ranga Dinesh, A.M. Savill, K.M. Jenkins, M.P. Kirkpatrick, Comput. Fluids 39, 1685–1695 (2010)
C.J. Lawn, Prog. Energy, Combust. Sci. 35, 1–30 (2009)
Acknowledgments
This research is being developed at the Federal University of Rio Grande do Sul—UFRGS. Andreis and Vaz thank the financial support from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES—Brazil, and Prof. De Bortoli gratefully acknowledges the financial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq—Brazil, under process 303007/2009-5
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andreis, G.S.L., Vaz, F.A. & De Bortoli, A.L. Bioethanol combustion based on a reduced kinetic mechanism. J Math Chem 51, 1584–1598 (2013). https://doi.org/10.1007/s10910-013-0166-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10910-013-0166-3