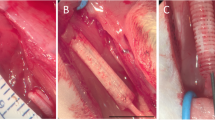
Abstract
Many studies have been dedicated to the development of scaffolds for improving post-traumatic nerve regeneration with different biomaterials. Nerve autografting is the most common surgical procedure currently used to repair nerve defects as a gold standard. To address the disadvantages of limited availability of donor nerves and donor site morbidity, we have fabricated chitosan conduits and seeded them combined with bone marrow mesenchymal stem cells (BMSCs) as an alternative. The conduits were tested for efficacy in bridging the critical gap (8 mm) in sciatic nerves of adult rats, which including sciatic nerve function index (SFI), ethology observation, histologic detection, immunohistochemistry detection. The BMSCs were tested for survival rate and differentiation by fluorescence labeling. Six weeks after operation, the SFI, average regenerated fiber density, and fiber diameter in nerves bridged with BMSCs were similar to those treated with autograft, but significantly higher than those bridged with chitosan conduits only (P < 0.05) because of the differentiation of BMSCs. Evidence is thus provided to support the effect of using multi-channel chitosan conduits seeded with BMSCs to treat critical defects in peripheral nerves. This provides the basis to pursue chitosan and BMSCs combination is an effective method to improve the nerve healing, which may be used as an alternative to the conventional nerve autografts.







Similar content being viewed by others
References
Schlosshauer B, Dreesmann L, Schaller HE, Sinis N. Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery. 2006;59:740–7.
Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347.
Rose NR, Burek CL. The interaction of basic science and population-based research: autoimmune thyroiditis as a case history. Am J Epidemiol. 1991;134:1073–8.
Belkas JS, Shoichet MS, Midha R. Peripheral nerve regeneration through guidance tubes. Neurol Res. 2004;26:151–60.
Samii M, Carvalho GA, Nikkhah G, Penkert G. Surgical reconstruction of the musculocutaneous nerve in traumatic brachial plexus injuries. J Neurosurg. 1997;87:881–6.
Lundborg G. Enhancing post-traumatic nerve regeneration. J Peripher Nerv Syst. 2002;7:139–40.
Geuna S, Papalia I, Tos P. End-to-side (terminolateral) nerve regeneration: a challenge for neuroscientists coming from an intriguing nerve repair concept. Brain Res Rev. 2006;52:381–8.
Hoke A. Mechanisms of disease: What factors limit the success of peripheral nerve regeneration in humans? Nat Clin Pract Neurol. 2006;2:448–54.
Tanigawa J, Miyoshi N, Sakurai K. J Appl Polym Sci. 2008;110:608–15.
Chalfoun CT, Wirth GA, Evans GR. Tissue engineered nerve constructs: Where do we stand? J Cell Mol Med. 2006;10:309–17.
Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials. 2003;24:2339–49.
Maorong J, Xiaoming Z, Yumin Y, Xiaosong G, Fei D. The promotion of peripheral nerve regeneration by chitooligosaccharides in the rat nerve crush injury model. Neurosci Lett. 2009;454:239–43.
Siemionow M, Bozkurt M, Zor F. Regeneration and repair of peripheral nerves with different biomaterials: review. Microsurgery. 2010;30:574–88.
Ciardelli G, Chiono V. Materials for peripheral nerve regeneration. Macromol Biosci. 2005;6:13–26.
Amado S, Simoes MJ, Armada da Silva PA, Luís AL, Shirosaki Y, Lopes MA, Santos JD, Fregnan F, Gambarotta G, Raimondo S, Fornaro M, Veloso AP, Varejão AS, Maurício AC, Geuna S. Use of hybrid chitosan membranes and N1E-115 cells for promoting nerve regeneration in an axonotmesis rat model. Biomaterials. 2008;29:4409–19.
Pountos I, Corscadden D, Emery P, Giannoudis PV. Mesenchymal stem cell tissue engineering: techniques for isolation, expansion and application. Injury. 2007;38:S23–33.
Brehm M, Zeus T, Strauer BE. Stem cells—clinical application and perspectives. Herz. 2002;27:611–20.
Zheng L, Cui HF. Use of chitosan conduit combined with bone marrow mesenchymal stem cells for promoting peripheral nerve regeneration. J Mater Sci Mater Med. 2010;21:1713–20.
Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129–36.
Ao Q, Fung CK, Tsui AY, Cai S, Zuo HC, Chan YS, Shum DK. The regeneration of transected sciatic nerves of adult rats using chitosan nerve conduits seeded with bone marrow stromal cell-derived Schwann cells. Biomaterials. 2011;32:787–96.
Rosales-Cortés M, Peregrina-Sandoval J, Bañuelos-Pineda J, Sarabia-Estrada R, Gómez-Rodiles CC, Albarrán-Rodríguez E, Zaitseva GP, Pita-López ML. Immunological study of a chitosan prosthesis in the sciatic nerve regeneration of the axotomized dog. J Biomater Appl. 2003;18:15–23.
Itoh S, Yamaguchi I, Suzuki M, Ichinose S, Takakuda K, Kobayashi H, Shinomiya K, Tanaka J. Hydroxyapatite-coated tendon chitosan tubes with adsorbed laminin peptides facilitate nerve regeneration in vivo. Brain Res. 2003;993:111–23.
Ishikawa N, Suzuki Y, Ohta M, Cho H, Suzuki S, Dezawa M, Ide C. Peripheral nerve regeneration through the space formed by a chitosan gel sponge. J Biomed Mater Res A. 2007;83:33–40.
Patel M, Vandevord PJ, Matthew H, Wu B, DeSilva S, Wooley PH. Video-gait analysis of functional recovery of nerve repaired with chitosan nerve guides. Tissue Eng. 2006;12:3189–99.
Wang A, Ao Q, Cao W, Yu M, He Q, Kong L, Zhang L, Gong Y, Zhang X. Porous chitosan tubular scaffolds with knitted outer wall and controllable inner structure for nerve tissue engineering. J Biomed Mater Res A. 2006;79:36–46.
Moore MJ, Friedman JA, Lewellyn EB, Mantila SM, Krych AJ, Ameenuddin S, Knight AM, Lu L, Currier BL, Spinner RJ, Marsh RW, Windebank AJ, Yaszemski MJ. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials. 2006;27:419–29.
Aijun W, Qiang A, Qing H, Xiaoming G, Kai G, Yandao G, Nanming Z, Xiufang Z. Neural stem cell affinity of chitosan and feasibility of chitosan-based porous conduits as scaffolds for nerve tissue engineering. Tsinghua Sci Technol. 2006;11:415–20.
Wenling C, Duohui J, Jiamou L, Yandao G, Nanming Z, Xiufang Z. Effects of the degree of deacetylation on the physicochemical properties and Schwann cell affinity of chitosan films. J Biomater Appl. 2005;20:157–77.
Hsu S-h, Whu SW, Tsai C-L, Wu Y-H, Chen H-W, Hsieh K-H. Chitosan as scaffold materials: effects of molecular weight and degree of deacetylation. J Polym Res. 2004;11:141–7.
Freier T, Koh HS, Kazazian K, Shoichet MS. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials. 2005;26:5872–8.
Zhao L, Lin Y, Ma J, Sun Y, Zeng S, Zhang X, Zuo M. Culture and neural differentiation of rat bone marrow mesenchymal stem cells in vitro. Cell Biol Int. 2007;31:916–23.
Wislet-Gendebien S, Leprince P, Moonen G, Rogister B. Regulation of neural markers nestin and GFAP expression by cultivated bone marrow stromal cells. J Cell Sci. 2003;116:3295–302.
Wislet-Gendebien S, Hans G, Leprince P, Rigo JM, Moonen G, Rogister B. Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem Cells. 2005;23:392–402.
Wang J, Ding F, Gu Y, Liu J, Gu X. Bone marrow mesenchymal stem cells promote cell proliferation and neurotrophic function of Schwann cells in vitro and in vivo. Brain Res. 2009;1262:7–15.
Li J, Gong Y, Zhao N, Zhang X. Preparation of N-butyl chitosan and study of its physical and biological properties. J Appl Polym Sci. 2005;98:1016–24.
Evans GR, Facs MD. Challenges to nerve regeneration. Semin Surg Oncol. 2000;19:312–8.
Lu L, Chen X, Zhang CW, Yang WL, Wu YJ, Sun L, Bai LM, Gu XS, Ahmed S, Dawe GS, Xiao ZC. Morphological functional characterization of predifferentiation of myelinating glia-like cells from human bone marrow stromal cells through activation of F3/Notch signaling in mouse retina. Stem Cells. 2008;26:580–90.
Lu J, Moochhala S, Moore XL, Ng KC, Tan MH, Lee LK, He B, Wong MC, Ling EA. Adult bone marrow cells differentiate into neural phenotypes improve functional recovery in rats following traumatic brain injury. Neurosci Lett. 2006;398:12–7.
Yang Y, Chen X, Ding F, Zhang P, Liu J, Gu X. Biocompatibility evaluation of silk fibroin with peripheral nerve tissues cells in vitro. Biomaterials. 2007;28:1643–52.
Wang X, Hu W, Cao Y, Yao J, Wu J, Gu X. Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain. 2005;128:1897–910.
Cheng M, Cao W, Gao Y, Gong Y, Zhao N, Zhang X. Studies on nerve cell affinity of biodegradable modified chitosan films. J Biomater Sci Polym Ed. 2003;14:1155–67.
Cheng MY, Deng JG, Yang F, Gong YD, Zhao NM, Zhang XF. Study on physical properties and nerve cell affinity of composite films from chitosan and gelatin solutions. Biomaterials. 2003;24:2871–80.
Yuan Y, Zhang P, Yang Y, Wang X, Gu X. The interaction of Schwann cells with chitosan membranes and fibers in vitro. Biomaterials. 2004;25:4273–8.
Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999;96:10711–6.
Ernst C, Christie BR. The putative neural stem cell marker, nestin, is expressed in heterogeneous cell types in the adult rat neocortex. Neuroscience. 2006;138:183–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, L., Cui, HF. Enhancement of nerve regeneration along a chitosan conduit combined with bone marrow mesenchymal stem cells. J Mater Sci: Mater Med 23, 2291–2302 (2012). https://doi.org/10.1007/s10856-012-4694-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-012-4694-3