Abstract
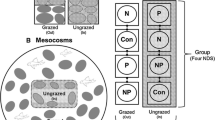
Since periphytic biofilm is an important source of food in lotic ecosystems, it is important to understand how key ecological factors affect the accrual and loss of algal biomass and sediment in the biofilm. We designed a field experiment to evaluate the effects of mesohabitat type (pools and riffles), grazing fish (control and exclusion), and substrate roughness (smooth and rough) on chlorophyll a, ash-free dry mass (AFDM), and total dry mass in a subtropical stream. Mesohabitat type did not influence the effect of grazers on periphyton. However, rough substrates accumulated more total dry mass in pools than in riffles, while smooth substrates accumulated similar amounts of total dry mass in both mesohabitats. The accrual of AFDM and chlorophyll a was greater on rough than on smooth substrates, regardless of mesohabitat. Treatments without fish accrued more total dry mass, AFDM, and chlorophyll a than treatments with fish, showing that fish play a major role in this stream by removing sediment and algal biomass. These results suggest that habitat simplification in the scale of substrate roughness and loss of large grazers may impact the accrual and loss of algal biomass and sediment in lotic ecosystems.

Similar content being viewed by others
References
Abe, S., K. Uchida, T. Nagumo & J. Tanaka, 2007. Alterations in the biomass-specific productivity of periphyton assemblages mediated by fish grazing. Freshwater Biology 52: 1486–1493.
Allan, J. D. & M. M. Castillo, 2007. Stream Ecology, 2nd ed. Springer, Dordrecht.
Álvarez, M. & B. L. Peckarsky, 2005. How do grazers affect periphyton heterogeneity in streams? Oecologia 142: 576–587.
Barbee, N., 2005. Grazing insects reduce algal biomass in a neotropical stream. Hydrobiologia 532: 153–165.
Behling, H., 2002. South and southeast Brazilian grasslands during Late Quaternary times: a synthesis. Palaeogeography, Palaeoclimatology, Palaeoecology 177: 19–27.
Bergey, E. A., 1999. Crevices as refugia for stream diatoms: effect of crevice size on abraded substrates. Limnology and Oceanography 44: 1522–1529.
Bergey, E. A., 2005. How protective are refuges? Quantifying algal protection in rock crevices. Freshwater Biology 50: 1163–1177.
Bergey, E. A. & J. E. Weaver, 2004. The influence of crevice size on the protection of epilithic algae from grazers. Freshwater Biology 49: 1014–1025.
Bertrand, K. N. & K. B. Gido, 2007. Effects of the herbivorous minnow, southern redbelly dace (Phoxinus erythrogaster), on stream productivity and ecosystem structure. Oecologia 151: 69–81.
Biggs, B. J. F., 1996. Patterns in benthic algae of streams. In Stevenson, J., M. L. Bothwell & R. L. Lowe (eds), Algal Ecology: Freshwater Benthic Ecosystems. Elsevier, San Diego: 31–56.
Biggs, B. J. F. & C. Kilroy, 2000. Stream Periphyton Monitoring Manual. National Institute of Water and Atmospheric Research, Christchurch.
Bowen, S. H., 1983. Detritivory in Neotropical fish communities. Environmental Biology of Fishes 9: 137–144.
Buck, S. & I. Sazima, 1995. An assemblage of mailed catfishes (Loricariidae) in southeastern Brazil: distribution, activity, and feeding. Ichthyological Exploration of Freshwaters 6: 325–332.
Buckup, L., A. A. P. Bueno, G. Bond-Buckup, M. Casagrande & F. Majolo, 2007. The benthic macroinvertebrate fauna of highland streams in southern Brazil: composition, diversity and structure. Revista Brasileira de Zoologia 24: 294–301.
Cardinale, B. J., M. A. Palmer, C. M. Swan, S. Brooks & N. L. Poff, 2002. The influence of substrate heterogeneity on biofilm metabolism in a stream ecosystem. Ecology 83: 412–422.
Cross, W. F., A. Ramírez, A. Santana & L. Silvestrini-Santiago, 2008. Toward quantifying the relative importance of invertebrate consumption and bioturbation in Puerto Rican streams. Biotropica 40: 477–484.
Dias, T. S. & C. B. Fialho, 2011. Comparative dietary analysis of Eurycheilichthys pantherinus and Pareiorhaphis hystrix: two Loricariidae species (Ostariophysi, Siluriformes) from Campos Sulinos biome, southern Brazil. Iheringia, Serie Zoologia 101: 49–55.
Dudley, T. L. & C. M. D’Antonio, 1991. The effects of substrate texture, grazing, and disturbance on macroalgal establishment in streams. Ecology 72: 297–309.
Feminella, J. W. & C. P. Hawkins, 1995. Interactions between stream herbivores and periphyton: a quantitative analysis of past experiments. Journal of the North American Benthological Society 14: 465–509.
Flecker, A. S., 1996. Ecosystem engineering by a dominant detritivore in a diverse tropical stream. Ecology 77: 1845–1854.
Flecker, A. S., 1997. Habitat modification by tropical fishes: environmental heterogeneity and the variability of interaction strength. Journal of the North American Benthological Society 16: 286–295.
Flecker, A. S., B. P. Feifarek & B. W. Taylor, 1999. Ecosystem engineering by a tropical tadpole: density-dependent effects on habitat structure and larval growth rates. Copeia 1999: 495–500.
Jones, C. G., J. H. Lawton & M. Shachak, 1994. Organisms as ecosystem engineers. Oikos 69: 373–386.
Kovalenko, K. E., S. M. Thomaz & D. M. Warfe, 2012. Habitat complexity: approaches and future directions. Hydrobiologia 685: 1–17.
Lakatos, G., 1989. Composition of reed periphyton (biotecton) in the Hungarian part of Lake Fertö. BFB-Bericht 71: 125–134.
Landeiro, V. L., N. Hamada & A. S. Melo, 2008. Responses of aquatic invertebrate assemblages and leaf breakdown to macroconsumer exclusion in Amazonian “terra firme” streams. Fundamental and Applied Limnology 172: 49–58.
Lowe, R. L. & Y. Pan, 1996. Benthic algal communities as biological monitors. In Stevenson, J., M. L. Bothwell & R. L. Lowe (eds), Algal Ecology: Freshwater Benthic Ecosystems. Elsevier, San Diego: 705–739.
Moulton, T. P., M. L. Souza, R. M. L. Silveira & F. A. M. Krsulovic, 2004. Effects of ephemeropterans and shrimps on periphyton and sediments in a coastal stream (Atlantic forest, Rio de Janeiro, Brazil). Journal of the North American Benthological Society 23: 868–881.
Moulton, T. P., M. L. Souza, R. M. L. Silveira, F. A. M. Krsulovic, M. P. Silveira, J. C. F. Assis & C. N. Francischetti, 2010. Patterns of periphyton are determined by cascading trophic relationships in two neotropical streams. Marine and Freshwater Research 61: 57–64.
Moulton, T. P., M. L. Souza, E. F. Brito, M. R. A. Braga & S. E. Bunn, 2012. Strong interactions of Paratya australiensis (Decapoda: Atyidae) on periphyton. Marine and Freshwater Research 63: 834–844.
Murdock, J. N. & W. K. Dodds, 2007. Linking benthic algal biomass to stream substratum topography. Journal of Phycology 43: 449–460.
Opsahl, R. W., T. Wellnitz & N. L. Poff, 2003. Current velocity and invertebrate grazing regulate stream algae: results of an in situ electrical exclusion. Hydrobiologia 499: 135–145.
Padial, A. A., S. M. Thomaz & A. A. Agostinho, 2009. Effects of structural heterogeneity provided by the floating macrophyte Eichhornia azurea on the predation efficiency and habitat use of the small Neotropical fish Moenkhausia sanctaefilomenae. Hydrobiologia 624: 161–170.
Poff, N. L. & J. V. Ward, 1995. Herbivory under different flow regimes: a field experiment and test of a model with a benthic stream insect. Oikos 71: 179–188.
Power, M. E., 1990. Resource enhancement by indirect effects of grazers: armored catfish, algae, and sediment. Ecology 71: 897–904.
Power, M. E., W. J. Matthews & A. J. Stewart, 1985. Grazing minnows, piscivorous bass, and stream algae: dynamics of a strong interaction. Ecology 66: 1448–1456.
Pringle, C. M. & G. A. Blake, 1994. Quantitative effects of atyid shrimp (Decapoda: Atyidae) on the depositional environment in a tropical stream: use of electricity for experimental exclusion. Canadian Journal of Fisheries and Aquatic Sciences 51: 1443–1450.
Quintans, F., F. Scasso, M. Loureiro & A. Yafe, 2009. Diet of Cnesterodon decemmaculatus (Poeciliidae) and Jenynsia multidentata (anablepidae) in a hypertrophic shallow lake of Uruguay. Iheringia, Serie Zoologia 99: 99–105.
Rahel, F. J., 2000. Homogenization of fish faunas across the United States. Science 288: 854–856.
Ranvestel, A. W., K. R. Lips, C. M. Pringle, M. R. Whiles & R. J. Bixby, 2004. Neotropical tadpoles influence stream benthos: evidence for the ecological consequences of decline in amphibian populations. Freshwater Biology 49: 274–285.
Schneck, F. & A. S. Melo, 2013. High assemblage persistence in heterogeneous habitats: an experimental test with stream benthic algae. Freshwater Biology 58: 365–371.
Schneck, F., A. Schwarzbold & A. S. Melo, 2011. Substrate roughness affects stream benthic algal diversity, assemblage composition, and nestedness. Journal of the North American Benthological Society 30: 1049–1056.
Schwarzbold, A., 1990. Métodos ecológicos aplicados ao estudo do perifíton. Acta Limnologica Brasiliensia 3: 545–592.
Souza, M. L. & T. P. Moulton, 2005. The effect of shrimp on benthic material in a Brazilian island stream. Freshwater Biology 50: 592–602.
Steinman, A. D., 1996. Effects of grazers on freshwater benthic algae. In Stevenson, R. J., M. L. Bothwell & R. L. Lowe (eds), Algal Ecology: Freshwater Benthic Ecosystems. Elsevier, San Diego: 341–373.
Steinman, A. D., G. A. Lamberti & P. R. Leavitt, 2007. Biomass and pigments of benthic algae. In Hauer, R. & G. A. Lamberti (eds), Methods in Stream Ecology, 2nd ed. Elsevier, San Diego: 357–379.
Taniguchi, H. & M. Tokeshi, 2004. Effects of habitat complexity on benthic assemblages in a variable environment. Freshwater Biology 49: 1164–1178.
R Development Core Team, 2010. R. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. URL http://www.R-project.org/.
Winckler-Sosinski, L. T., A. Schwarzbold & U. H. Schultz, 2009. Fish assemblage structure in altitude rivers under the effect of exotic species introduction, northeast of Rio Grande do Sul, Brazil. Acta Limnologica Brasiliensia 21: 473–482.
Acknowledgments
We are grateful to Ana Pressi, Flávia Montagner, Geraldo Schneck, Marcelo Saraiva, Silvia Milesi, and Marlon Vasconcelos for invaluable field assistance, and to Victor Landeiro for suggestions regarding the construction of electrical fences. Sidinei M. Thomaz, Leandro Duarte, Luciane Crossetti, and two anonymous referees provided useful comments on the manuscript. FS received a student fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). ASM received research grants (476304/2007-5; 474560/2009-0) and a research fellowship (302482/2008-3) from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Katya E. Kovalenko
Rights and permissions
About this article
Cite this article
Schneck, F., Schwarzbold, A. & Melo, A.S. Substrate roughness, fish grazers, and mesohabitat type interact to determine algal biomass and sediment accrual in a high-altitude subtropical stream. Hydrobiologia 711, 165–173 (2013). https://doi.org/10.1007/s10750-013-1477-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1477-x