Abstract
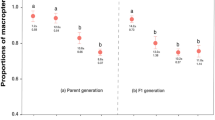
Evolution of costly secondary defences for a cryptic prey is puzzling, if the prey is already well protected by camouflage. However, if the chemical defence is not sufficient to deter all predators, selection can favour low signal intensity in defended prey. Alternatively, if the costs of chemical defence are low or cost-free, chemical defences can be expected to evolve also for non-signalling prey, particularly if conspicuous signalling is costly. We tested these assumptions with pine sawfly larvae (Neodiprion sertifer and Diprion pini) that are cryptically coloured and chemically defended with resin acids sequestered from their host plant (Pinus sp.). Larvae feed in large aggregations, which we hypothesise could function as a signal of unprofitability. Our results show that even though the birds found N. sertifer larvae unprofitable in the controlled laboratory assays, they continued attacking and consuming them in the wild. When we tested the signal value of aggregation we found that a large group size did not offer protection for a defended larva: the survival was higher in groups of 10 individuals compared to groups of 50, suggesting increased detectability costs for individuals in larger groups. Finally, we tested how costly the production and maintenance of a chemical defence is for D. pini larvae by manipulating the resin acid content of the diet. We did not find any life history or immunological costs of the chemical defence for the larvae. In contrast, pupal weights were higher on the high resin diet than on the low resin diet. Also, larvae were able to produce higher amounts of defence fluids on the high diet than on the low diet. Thus, our result suggests high detectability costs and low production costs of defences could explain why some unprofitable species have not evolved conspicuous signals.



Similar content being viewed by others
References
Alatalo R, Mappes J (1996) Tracking the evolution of warning signals. Nature 382:708–710
Aldrich JR, Blum MS (1978) Aposematic aggregation of a bug (Hemiptera: Coreidae): The defensive display and formation of aggregations. Biotropica 10:58–61
Aukema BH, Raffa KF (2004) Does aggregation benefit bark beetles by diluting predation? Links between a group-colonisation strategy and the absence of emergent multiple predator effects. Ecol Entomol 29:129–138
Barnett CA, Bateson M, Rowe C (2007) State-dependent decision making: educated predators strategically trade off the costs and benefits of consuming aposematic prey. Behav Ecol 18:645–651
Beatty CD, Roderick SB, Sherratt TN (2005) The evolution of aggregation in profitable and unprofitable prey. Anim Behav 70:199–208
Björkman C, Larsson S (1991) Pine sawfly defence and variation in host plant resin acids: a trade-off with growth. Ecol Entomol 16:283–289
Björkman C, Larsson S, Gref R (1991) Effects of nitrogen fertilization on pine needle chemistry and sawfly performance. Oecologia 86:202–209
Björkman C, Larsson S, Bommarco R (1997) Oviposition preferences in pine sawflies: a trade-off between larval growth and defence against natural enemies. Oikos 79:45–52
Blount JD, Speed MP, Ruxton G, Stephens PA (2009) Warning displays may function as honest signals of toxicity. Proc R Soc Lond B 276:871–877
Camara MD (1997) Physiological mechanisms underlying the costs of chemical defence in Junonia coenia Hübner (Nymphalidae): a gravimetric and quantitative genetic analysis. Evol Ecol 11:451–469
Casey TM, Knapp R (1987) Caterpillar thermal adaptation: behavioural differences reflect metabolic thermal sensitivities. Comp Biochem Physiol 86A:679–682
Codella SG, Raffa KF (1995a) Host plant influence on chemical defense in conifer sawflies (Hymenoptera: Diprionidae). Oecologia 104:1–11
Codella SG, Raffa KF (1995b) Contributions of female oviposition patterns and larval behaviour to group defense in conifer sawflies (Hymenoptera: Diprionidae). Oecologia 103:24–33
Codella SG, Raffa KF (1996) Individual and social components of wood ant response to conifer sawfly defence (Hymenoptera: Formicidae, Diprionidae). Anim Behav 52:801–811
Cook JM (1997) Sex determination in the Hymenoptera: a review of models and evidence. Heredity 71:421–435
Cott HB (1940) Adaptive colouration in animals. Methuen, London
Cotter SC, Hails RS, Cory JS, Wilson K (2004) Density-dependent prophylaxis and condition-dependent immune function in Lepidopteran larvae: a multivariate approach. J Anim Ecol 73:283–293
Darst CR, Cummings ME, Cannatella DC (2006) A mechanism for diversity in warning signals: conspicuousness versus toxicity in poison frogs. PNAS 103:5852–5857
Del Campo M, Smedley SR, Eisner T (2005) Reproductive benefits derived from defensive plant alkaloid possession in an Arctiid moth (Utetheisa ornatrix). PNAS 102:13508–13512
Eisner T, Johnessee JS, Carrel J (1974) Defensive use by an insect of a plant resin. Science 184:996–999
Endler JA (1991) Interactions between predators and prey. In: Krebs JR, Davies NB (eds) Behavioural ecology. An evolutionary approach. Blackwell Science, Cambridge, pp 169–196
Endler JA, Mappes J (2004) Predator mixes and the conspicuousness of aposematic signals. Am Nat 163:532–547
Exnerová A, Svadová K, Štys P, Barcalová S, Landová E, Prokopová M, Fuchs R, Socha R (2006) Importance of colour in the reaction of passerine predators to aposematic prey: experiments with mutants of Pyrrhocoris apterus (Heteroptera). Biol J Linn Soc 88:143–153
Fischer S, Samietz J, Wäckers FL, Dorn S (2001) Interaction of vibrational and visual cues in parasitoid host location. J Comp Physiol A 187:785–791
Forsman A, Merilaita S (1999) Fearful symmetry: pattern size and asymmetry affects aposematic signal efficacy. Evol Ecol 13:131–140
Friman V, Lindstedt C, Hiltunen T, Laakso J, Mappes J (2009) Predation on multiple trophic levels shapes the evolution of pathogen virulence. PLoS ONE 4(8):e6761
Gagliardo A, Guilford T (1993) Why do warning coloured prey live gregariously? Proc R Soc Lond Ser B 286:149–150
Gamberale-Stille G (2000) Decision time and prey gregariousness influence attack probability in naive and experienced predators. Anim Behav 60:95–99
Gamberale G, Sillén-Tullberg B (1998) Aposematism and gregariousness: the combined effect of group size and coloration on signal repellence. Proc R Soc Lond B 265:889–894
Gamberale G, Tullberg BS (1996) Evidence for a more effective signal in aggregated aposematic prey. Anim Behav 52:597–601
Gamberale-Stille G, Tullberg B (1999) Experienced chicks show biased avoidance of stronger signals: an experiment with natural colour variation in live aposematic prey. Evol Ecol 13:579–589
Gentry GL, Dyer LA (2002) On the conditional nature of Neotropical caterpillar defences against their natural enemies. Ecology 83:3108–3119
Grill CP, Moore AJ (1998) Effects of a larval antipredator response and larval diet on adult phenotype in an aposematic ladybird beetle. Oecologia 114:274–282
Hagman M, Forsman A (2003) Correlated evolution of conspicuous colouration and body size in poison frogs (Dendrobatidae). Evolution 57:2904–2910
Ham AD, Ihalainen E, Lindström L, Mappes J (2006) Does colour matter? The importance of colour in avoidance learning, memorability and generalisation. Behav Ecol Sociobiol 60:482–491
Harvey JA, van Nouhuys S, Biere A (2005) Effects of quantitative variation in allelochemicals in Plantago lanceolata on development of a generalist and a specialist herbivore and their endoparasitoids. J Chem Ecol 31:287–302
Hedlund K, Vet LEM, Dicke M (1996) Generalist and specialist parasitoid strategies of using odours of adult drosophilid flies when searching for larval hosts. Oikos 77:390–398
Heimpel GE, de Boer JG (2008) Sex determination in Hymenoptera. Annu Rev Entomol 53:209–230
Herz A, Heitland W (1999) Larval parasitism of a forest pest, the common pine sawfly Diprion pini (L.) (HYM., Diprionidae), during an endemic density phase. J Appl Ent 123:129–137
Holloway GJ, de Jong P, Brakefield PM, de Vos H (1991) Chemical defence in ladybird beetles (Coccinellidae). I. Distribution of coccinelline and individual variation in defence in 7-spot ladybirds (Coccinella septempunctata). Chemoecology 2:7–14
Holloway GJ, de Jong PW, Ottenheim M (1993) The genetics and cost of chemical defence in the 2-spot ladybird (Adalia bipunctata L.). Evolution 47:1229–1239
Holloway GJ, Gilbert F, Brandt A (2001) The relationship between mimetic imperfection and phenotypic variation in insect colour patterns. Proc R Soc Lond B 269:411–416
Hunter AF (2000) Gregariousness and repellent defences in the survival of phytophagous insects. Oikos 91:213–224
Kalin M, Knerer G (1977) Group and mass effects in diprionid sawflies. Nature 267:427–429
Klemola N, Klemola T, Rantala MJ, Ruuhola T (2007) Natural host-plant quality affects immune defence of an insect herbivore. Entomol Exp Appl 123:167–176
Koskimäki J, Rantala MJ, Taskinen J, Tynkkynen K, Suhonen J (2004) Immunocompetence and resource holding potential in the damselfly, Calopteryx virgo L. Behav Ecol 15:169–173
Larsson S, Björkman C, Gref R (1986) Responses of Neodiprion sertifer (Hym., Diprionidae) larvae to variation in needle resin acid concentration in Scots pine. Oecologia 70:77–84
Larsson S, Lundgren L, Ohmart CP, Gref R (1992) Weak responses of pine sawfly larvae to high needle flavonoid concentrations in scots pine. J Chem Ecol 18:271–282
Larsson S, Ekbom B, Björkman C (2000) Influence of plant quality on pine sawfly population dynamics. Oikos 89:440–450
Lawrence WS (1990) The efects of group size and host species on development and survivorship of a gregarious caterpillar Halisidota caryae (Lepidoptera: Arctiidae). Eco Entomol 15:53–62
Leimar O, Enquist M, Sillén-Tullberg B (1986) Evolutionary stability of aposematic coloration and prey unprofitability: a theoretical analysis. Am Nat 128:469–490
Lindstedt C, Mappes J, Päivinen J, Varama M (2006) Effects of group size and pine defence chemicals on Diprionid sawfly survival against ant predation. Oecologia 150:519–526
Lindstedt C, Lindström L, Mappes J (2008) Hairiness and warning colours as components of antipredator defence: additive or interactive benefits? Anim Behav 75:1703–1713
Lindstedt C, Lindström L, Mappes J (2009a) Thermoregulation constrains effective warning signal expression. Evolution 63:469–478
Lindstedt C, Reudler Talsma J, Ihalainen E, Lindström L, Mappes J (2009b) Diet quality affects coloration indirectly: excretion costs in a generalist herbivore. Evolution 64:68–78
Lindström L, Alatalo R, Mappes J, Riipi M, Vertainen L (1999) Can aposematic signals evolve by gradual change? Nature 397:249–251
Lindström L, Alatalo R, Lyytinen A, Mappes J (2001) Strong antiapostatic selection against novel rare aposematic prey. PNAS 98:9181–9184
Lindström L, Alatalo RV, Lyytinen A, Mappes J (2004) The effect of alternative prey on the dynamics of Batesian and Müllerian mimicries. Evolution 58:1294–1302
Longson CG, Joss JMP (2006) Optimal toxicity in animals: predicting the optimal level of chemical defences. Func Ecol 20:731–735
Mappes J, Alatalo RV (1997) Effect of novelty and gregariousness in survival of aposematic prey. Behav Ecol 8:174–177
Mappes J, Marples N, Endler J (2005) The complex business of survival by aposematism. TREE 20:598–603
Marshall NJ (2000) Communication and camouflage with the same ‘bright’ colours in reef fishes. Phil Trans R Soc B 355:1243–1248
Mattiacci L, Hütter E, Schoch D, Scascighini N, Dorn S (2000) Plant-odour mediates parasitoid host handling and oviposition in an endophytic tritrophic system. Chemoecology 10:185–192
Merilaita S, Kaitala V (2002) Community structure and the evolution of aposematic colouration. Ecol Lett 5:495–501
Merilaita S, Ruxton G (2007) Aposematic signals and the relationship between conspicuousness and distinctiveness. J Theor Biol 245:268–287
Mopper S, Whitham TG, Price PW (1990) Plant phenotype and interspecific competition between insects determine sawfly performance and density. Ecology 71(6):2135–2144
Nilsson M, Forsman A (2003) Evolution of conspicuous colouration, body size and gregariousness: a comparative analysis of Lepidopteran larvae. Evol Ecol 17:51–66
Ojala K, Julkunen-Tiitto R, Lindström L, Mappes J (2005) Diet affects the immune defence and life-history traits of an Arctiid moth Parasemia plantaginis. Evol Ecol Res 7:1153–1170
Ojala K, Lindström L, Mappes J (2007) Life history constraints and warning signal expression in arctiid moth. Func Ecol 21:1162–1167
Poulton EB (1890) The colours of animals: their meaning and use especially considered in the case of insects (Edn 2), xiii, Kegan Paul, Trench, Trubner and co., London
Powell W, Pennacchio F, Poppy GM, Tremblay E (1998) Strategies involved in the location of hosts by the parasitoid Aphidius ervi Haliday (Hymenoptera: Braconidae: Aphidiinae). Biol Control 11:104–112
Prop N (1960) Protection against birds and parasites in some species of tenthredinid larvae. Arch Neerl Zool 13:380–447
Rantala MJ, Koskimäki J, Taskinen J, Tynkkynen K (2000) Immunocompetence developmental stability and wing spot size in Calopteryx splendens L. Proc R Soc Lond B 267:2453–2457
Reader T, Hochuli DF (2003) Understanding gregariousnees in a larval lepidoteran: the roles of host plant, predation and microclimate. Ecol Ent 28(6):729–737
Riipi M, Alatalo R, Lindström L, Mappes J (2001) Multiple benefits of gregariousness cover detectability costs in aposematic aggregations. Nature 413:512–514
Roque-Albelo L, Schroeder FC, Conner WE, Bezzerides A, Hoebeke ER, Meinwald J, Eisner T (2002) Chemical defence and aposematism: the case of Utetheisa galapagensis. Chemoecology 12:153–157
Rowe C, Lindström L, Lyytinen A (2004) The importance of pattern similarity between Müllerian mimics in predator avoidance learning. Proc R Soc Lond B 271:407–413
Rowland HM, Ihalainen E, Lindström L, Mappes J, Speed MP (2007) Co-mimics have a mutualistic relationship despite unequal defence levels. Nature 448:64–66
Ruxton GD, Sherrat TN (2006) Aggregation, defence and warning signals: the evolutionary relationship. Proc R Soc Lond B 273:2417–2424
Ruxton GD, Sherratt TN, Speed MP (2004) Avoiding attack. Evolutionary ecology of crypsis, warning signals and mimicry. Oxford University Press, New York
Ruxton GD, Speed MP, Broom M (2009) Identifying the ecological conditions that select for intermediate levels of aposematic signalling. Evol Ecol 23:491–501
Ryder JJ, Siva-Jothy MT (2000) Male calling song provides a reliable signal of immune function in a cricket. Proc R Soc Lond B 267:1171–1175
Saastamoinen M, van Nouhuys S, Nieminen M, O’Hara B, Suomi J (2007) Development and survival of a specialist herbivore, Melitaea cinxia, on host plants producing high and low concentrations of iridoid glycosides. Ann Zool Fenn 44:70–80
Sandre SL, Stevens M, Mappes J (2010) The effect of predator appetite, prey warning coloration and luminance on predator foraging decisions. Behaviour 147:1121–1143
Seymour RS (1979) Convective and evaporative cooling in sawfly larvae. J Insect Physiol 20:2447–2457
Sherratt TN, Beatty CD (2003) The evolution of warning signals as reliable indicators of prey defence. Am Nat 162:377–389
Sillén-Tullberg B (1985) Higher survival of an aposematic than of a cryptic form of a distasteful bug. Oecologia 67:411–415
Sillén-Tullberg B (1990) Do predators avoid groups of aposematic prey? An experimental test. Anim Behav 40:856–860
Sillén-Tullberg B, Leimar O (1988) The evolution of gregariousness in distasteful insects as a defence against predators. Am Nat 132:723–734
Skelhorn J, Rowe C (2006a) Prey palatability influences predator learning and memory. Anim Behav 71:1111–1118
Skelhorn J, Rowe C (2006b) Predator avoidance learning of prey with secreted or stored defences and the evolution of insect defences. Anim Behav 72:827–834
Skelhorn J, Ruxton GD (2006) Avian predators attack aposematic prey more forcefully when they are part of an aggregation. Biol Lett 2:488–490
Skelhorn J, Griksaitis D, Rowe C (2008) Colour biases are more than a question of taste. Anim Behav 75:827–835
Speed M, Ruxton G (2007) How bright and how nasty: explaining diversity in warning signal strength. Evolution 61:623–635
Speed M, Brockhurst MA, Ruxton GD (2010) Dual benefits of aposematism: predator avoidance and enhanced resource collection. Evolution 23:207–211
Stevens M, Merilaita S (2009) Defining disruptive coloration and distinguishing its functions. Phil Trans R Soc B 364:481–488
Tullberg B, Leimar O, Gamberale-Stille G (2000) Did aggregation favour the initial evolution of warning coloration? A novel world revisited. Anim Beh 59:281–287
Tullberg BS, Merilaita S, Wiklund C (2005) Aposematism and crypsis combined as a result of distance dependence: functional versatility of the colour pattern in the swallowtail butterfly larva. Proc R Soc Lond B 272:1315–1321
Turner JRG, Speed MP (1999) How weird can mimicry get? Evol Ecol 13:807–827
Wilson K, Reeson AS (1998) Density-dependent prophylaxis: evidence from Lepidoptera-baculovirus interactions? Ecol Entomol 23:100–101
Acknowledgments
We thank UPM Kymmene, Stora Enso and landowners in Konnevesi-area for provision of study areas. We also thank Sheena Cotter who kindly commented the text and Ville Friman for helping with analyses. This study was financed by the Academy of Finland (Finnish Centres of Excellence in Evolutionary Ecology and Evolutionary Research), Societas Biologica Fennica Vanamo 2002 and Luonnon Ystäväin Yhdistys ry of Kuopio.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lindstedt, C., Huttunen, H., Kakko, M. et al. Disengtangling the evolution of weak warning signals: high detection risk and low production costs of chemical defences in gregarious pine sawfly larvae. Evol Ecol 25, 1029–1046 (2011). https://doi.org/10.1007/s10682-010-9456-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-010-9456-4