Abstract
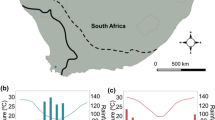
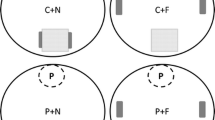
Species with a wide distribution over latitudinal gradients often exhibit increasing growth and development rates towards higher latitudes. Ecological theory predicts that these fast-growing genotypes are, in the absence of trade-offs with fast growth, better competitors than low-latitude conspecifics. While knowledge on key ecological traits along latitudinal clines is important for understanding how these clines are maintained, the relative competitive ability of high latitude individuals against low latitude conspecifics has not been tested. Growth and development rates of the common frog Rana temporaria increase along the latitudinal gradient across Scandinavia. Here we investigated larval competition over food resources within and between two R. temporaria populations originating from southern and northern Sweden in an outdoor common garden experiment. We used a factorial design, where southern and northern tadpoles were reared either as single populations or as mixes of the two populations at two densities and predator treatments (absence and non-lethal presence of Aeshna dragonfly larvae). Tadpoles from the high latitude population grew and developed faster and in the beginning of the experiment they hid less and were more active than tadpoles from the low latitude population. When raised together with high latitude tadpoles the southern tadpoles had a longer larval period, however, the response of high latitude tadpoles to the competition by low latitude tadpoles did not differ from their response to intra-population competition. This result was not significantly affected by density or predator treatments. Our results support the hypothesis that high latitude populations are better competitors than their low latitude conspecifics, and suggest that in R. temporaria fast growth and development trade off with other fitness components along the latitudinal gradient across Scandinavia.




Similar content being viewed by others
References
Altwegg R (2002) Predator-induced life-history plasticity under time constraints in pool frogs. Ecology 83:2542–2551
Altwegg R, Reyer H-U (2003) Patterns of natural selection on size at metamorphosis in water frogs. Evol Int J Org Evol 57:872–882
Angilletta MJ Jr, Wilson RS, Navas CA, James RS (2003) Tradeoffs and the evolution of thermal reaction norms. Trends Ecol Evol 18:234–240. doi:10.1016/S0169-5347(03)00087-9
APHA (1985) Standard methods for the examination of water and wastewater, 16th edn. American Public Health Association, Washington
Arendt JD (1997) Adaptive intrinsic growth rates: an integration across taxa. Q Rev Biol 72:149–177. doi:10.1086/419764
Arnett AE, Gotelli NJ (1999) Geographic variation in life-history traits of the ant lion, Myrmeleon immaculatus: evolutionary implications of Bergmann’s rule. Evol Int J Org Evol 53:1180–1188. doi:10.2307/2640821
Ashton KG (2004) Sensitivity of intraspecific latitudinal clines of body size for tetrapods to sampling, latitude and body size. Integr Comp Biol 44:403–412. doi:10.1093/icb/44.6.403
Berven KA (1990) Factors affecting population fluctuations in larval and adult stages of the wood frog (Rana sylvatica). Ecology 71:1599–1608. doi:10.2307/1938295
Berven KA, Gill DE (1983) Interpreting geographic variation in life-history traits. Am Zool 23:85–97
Biek R, Funk CW, Maxell BA, Mills LS (2002) What is missing in amphibian decline research: insights from ecological sensitivity analysis. Conserv Biol 16:728–734. doi:10.1046/j.1523-1739.2002.00433.x
Biro PA, Abrahams MV, Post JR, Parkinson EA (2004) Predators select against high growth rates and risk-taking behaviour in domestic trout populations. Proc R Soc Lond B Biol Sci 271:2233–2237. doi:10.1098/rspb.2004.2861
Biro PA, Abrahams MV, Post JR, Parkinson EA (2006) Behavioural trade-offs between growth and mortality explain evolution of submaximal growth rates. J Anim Ecol 75:1161–1171. doi:10.1111/j.1365-2656.2006.01137.x
Blanckenhorn WU, Dermot M (2004) Bergmann and converse Bergmann latitudinal clines in arthropods: two ends of a continuum? Integr Comp Biol 44:413–424. doi:10.1093/icb/44.6.413
Brodin T, Johansson F (2004) Conflicting selection pressures on the growth/predation risk trade-off in a damselfly. Ecology 85:2927–2932. doi:10.1890/03-3120
Connell JH (1983) On the prevalence and relative importance of inter-specific competition: evidence from field experiments. Am Nat 122:661–696. doi:10.1086/284165
Conover DO, Present TMC (1990) Countergradient variation in growth rate: compensation for length of the growing season among Atlantic silversides from different latitudes. Oecologia 83:316–324
Conover DO, Schultz ET (1995) Phenotypic similarity and the evolutionary significance of countergradient variation. Trends Ecol Evol 10:248–252. doi:10.1016/S0169-5347(00)89081-3
Conover DO, Brown JJ, Ehtisham A (1997) Countergradient variation in growth of young striped bass (Morone saxatilis) from different latitudes. Can J Fish Aquat Sci 54:2401–2409. doi:10.1139/cjfas-54-10-2401
Devlin RH, Johnsson JI, Smailus DE, Biagi CA, Jonsson E, Björnsson BT (1999) Increased ability to compete for food by growth hormone-transgenic coho salmon Oncorhynchus kisutsch (Walbaum). Aquacult Res 30:479–482. doi:10.1046/j.1365-2109.1999.00359.x
Gasc JP, Cabela A, Crnobrnja-Isailovic J, Dolmen D, Grossenbacher K, Haffner P, Lescure J, Martens H, Martinéz Rica JP, Oliveira ME, Sofianidou TS, Veith M, Zuiderwijk A (1997) Atlas of amphibians and reptiles in Europe. Societas Europaea Herpetologica and Muséum National d’Histoire Naturelle (IEGB/SPN), Paris
Gilchrist GW, Huey RB, Serra L (2001) Rapid evolution of wing size clines in Drosophila subobscura. Genetica 112–113:273–286. doi:10.1023/A:1013358931816
Gosner KN (1960) A simplified table for staging anuran embryos and larvae with notes of identification. Herpetologica 16:183–190
Grill CP, Juliano SA (1996) Predicting species interactions based on behaviour: predation and competition in container dwelling mosquitoes. J Anim Ecol 65:63–76. doi:10.2307/5700
Hellriegel B (2000) Single-or multistage regulation in complex life cycles: does it make a difference? Oikos 88:239–249. doi:10.1034/j.1600-0706.2000.880202.x
Imsland AK, Foss A, Nævdal G, Cross T, Bonga SW, Ham EV, Stefansson SO (2000) Countergradient variation in growth and food conversion efficiency of juvenile turbot. J Fish Biol 57:1213–1226. doi:10.1111/j.1095-8649.2000.tb00482.x
James AC, Partridge L (1998) Geographic variation in competitive ability in Drosophila melanogaster. Am Nat 151:530–537. doi:10.1086/286138
Johansson F, Stoks R, Rowe L, De Block M (2001) Life history plasticity in a damselfly: effects of combined time and biotic constraints. Ecology 82:1857–1869
Johansson M, Primmer CR, Merilä J (2006) History vs. current demography: explaining the genetic population structure of the common frog (Rana temporaria). Mol Ecol 15:975–983. doi:10.1111/j.1365-294X.2006.02866.x
Lankford TE Jr, Billerbeck JM, Conover DO (2001) Evolution of intrinsic growth and energy acquisition rates. II. Trade-offs in vulnerability to predation in Menidia menidia. Evol Int J Org Evol 55:1873–1881. doi:10.1111/j.0014-3820.2001.tb00836.x
Laugen AT, Laurila A, Merilä J (2002) Maternal and genetic contributions to geographical variation in Rana temporaria larval life-history traits. Biol J Linn Soc Lond 76:61–70. doi:10.1111/j.1095-8312.2002.tb01714.x
Laugen AT, Laurila A, Räsänen K, Merilä J (2003) Latitudinal countergradient variation in the common frog (Rana temporaria) development rates—evidence for local adaptation. J Evol Biol 16:996–1005. doi:10.1046/j.1420-9101.2003.00560.x
Laugen AT, Kruuk LEB, Laurila A, Räsänen K, Stone J, Merilä J (2005) Quantitative genetics of larval life-history traits in Rana temporaria in different environmental conditions. Genet Res 86:161–170. doi:10.1017/S0016672305007810
Laurila A, Pakkasmaa S, Merilä J (2001) Influence of seasonal time constraints on growth and development of common frog tadpoles: a photoperiod experiment. Oikos 95:451–460. doi:10.1034/j.1600-0706.2001.950310.x
Laurila A, Järvi-Laturi M, Pakkasmaa S, Merilä J (2004) Temporal variation in predation risk: stage-dependency, graded responses and fitness costs in tadpole antipredator defences. Oikos 107:90–99. doi:10.1111/j.0030-1299.2004.13126.x
Laurila A, Pakkasmaa S, Merilä J (2006) Population divergence in growth rate and antipredator defenses in Rana arvalis. Oecologia 147:585–595. doi:10.1007/s00442-005-0301-3
Laurila A, Lindgren B, Laugen AT (2008) Antipredator defenses along a latitudinal gradient in Rana temporaria. Ecology 89:1399–1413. doi:10.1890/07-1521.1
Lindgren B, Laurila A (2005) Proximate causes of adaptive growth rates: growth efficiency variation among latitudinal populations of Rana temporaria. J Evol Biol 18:820–828. doi:10.1111/j.1420-9101.2004.00875.x
Loman J (2004) Density regulation in tadpoles of Rana temporaria: a full pond field experiment. Ecology 85:1611–1618. doi:10.1890/03-0179
Mangel M, Stamps J (2001) Trade-offs between growth and mortality and the maintenance of individual variation in growth. Evol Ecol Res 3:583–593
Merilä J, Laurila A, Laugen AT, Räsänen K, Pahkala M (2000) Plasticity in age and size at metamorphosis in Rana temporaria—comparison of high and low latitude populations. Ecography 23:457–465. doi:10.1034/j.1600-0587.2000.230408.x
Merilä J, Laurila A, Lindgren B (2004) Variation in the degree and costs of adaptive phenotypic plasticity among Rana temporaria populations. J Evol Biol 17:1132–1140
Mousseau TA, Fox WF (1998) Maternal effects as adaptations. Oxford University Press, New York
Munch SB, Mangel M, Conover DO (2003) Quantifying natural selection in body size from field data: winter mortality in Menidia menidia. Ecology 84:2168–2177. doi:10.1890/02-0137
Odin H, Eriksson B, Perttu K (1983) Temperature climate maps for Swedish forestry. Department of Forest Soils, Swedish University of Agricultural Sciences, Uppsala
Orizaola G, Laurila A (2009) Microgeographic variation in temperature-induced plasticity in an isolated amphibian metapopulation. Evol Ecol (in press). doi:10.1007/s10682-008-9285-x
Palo JU, O’Hara RB, Laugen AT, Laurila A, Primmer CR, Merilä J (2003) Latitudinal divergence of common frog (Rana temporaria) life history traits by natural selection: evidence from a comparison of molecular and quantitative genetic data. Mol Ecol 12:1963–1968. doi:10.1046/j.1365-294X.2003.01865.x
Persson L (1985) Asymmetrical competition: are larger animals competitively superior? Am Nat 126:261–266. doi:10.1086/284413
Pidancier N, Gauthier P, Miquel C, Pompanon F (2001) Polymorphic microsatellite DNA loci identified in the common frog (Rana temporaria, Amphibia, Ranidae). Mol Ecol Notes 2:304–305. doi:10.1046/j.1471-8286.2002.00244.x
Räsänen K, Laurila A, Merilä J (2003) Geographic variation in acid stress tolerance of the moor frog, Rana arvalis. I. Local adaptation. Evol Int J Org Evol 57:352–362. doi:10.1554/0014-3820(2003)057[0352:GVIAST]2.0.CO;2
Relyea RA (2002) Local population differences in phenotypic plasticity: predator-induced changes in wood frog tadpoles. Ecol Monogr 72:77–93
Relyea RA (2007) Getting out alive: how predators affect the decision to metamorphose. Oecologia 152:389–400. doi:10.1007/s00442-007-0675-5
Roff DA (1980) Optimizing development time in a seasonal environment: the ‘ups and downs’ of clinal variation. Oecologia 45:202–208. doi:10.1007/BF00346461
Schoener TW (1983) Field experiments on inter-specific competition. Am Nat 122:240–285. doi:10.1086/284133
Sears MW (2005) Geographic variation in the life history of the sagebrush lizard: the role of thermal constraints on activity. Oecologia 143:25–36. doi:10.1007/s00442-004-1767-0
Skelly DK, Kiesecker JM (2001) Venue and outcome in ecological experiments: manipulations of larval anurans. Oikos 94:198–208. doi:10.1034/j.1600-0706.2001.t01-1-11105.x
Smith DC (1983) Factors controlling tadpole populations of the chorus frog (Pseudacris triseriata) on Isle Royale, Michigan. Ecology 64:501–510. doi:10.2307/1939970
Smith DC (1987) Adult recruitment in chorus frogs: effects of size and date and metamorphosis. Ecology 68:344–350. doi:10.2307/1939265
Uller T, Astheimer L, Olsson M (2007) Consequences of maternal yolk testosterone for offspring development and survival: experimental test in a lizard. Funct Ecol 21:544–551. doi:10.1111/j.1365-2435.2007.01264.x
Van Buskirk J, Arioli M (2005) Habitat specialization and adaptive divergence of anuran populations. J Evol Biol 18:596–608. doi:10.1111/j.1420-9101.2004.00869.x
Vonesh JR, De la Cruz O (2002) Complex life cycles and density dependence: assessing the contribution of egg mortality to amphibian declines. Oecologia 133:325–333. doi:10.1007/s00442-002-1039-9
Werner EE (1992) Competitive interactions between wood frog and northern leopard frog larvae: the influence of size and activity. Copeia 1992:26–35. doi:10.2307/1446532
Werner EE (1994) Ontogenetic scaling of competitive relations: size-dependent effects and responses in two anuran larvae. Ecology 75:197–213. doi:10.2307/1939394
Werner EE, Gilliam JF (1984) The ontogenetic niche shift and species interactions in size-structured populations. Annu Rev Ecol Syst 15:393–425. doi:10.1146/annurev.es.15.110184.002141
Wilbur HM (1980) Complex life cycles. Annu Rev Ecol Syst 11:67–93. doi:10.1146/annurev.es.11.110180.000435
Acknowledgments
We thank Sofia Wennberg for help with the experiment, Gunilla Engström and Kerstin Santesson for help in the molecular laboratory, and Jon Loman, Gerard Malsher, German Orizaola and Katja Räsänen for valuable comments on earlier versions of the manuscript. This study was performed with the permission of the Ethical Committee for Animal Experiments in Uppsala County and funded by the Swedish Research Council (grant to AL) and Zoologiska Stiftelsen (BL).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lindgren, B., Laurila, A. Are high-latitude individuals superior competitors? A test with Rana temporaria tadpoles. Evol Ecol 24, 115–131 (2010). https://doi.org/10.1007/s10682-009-9294-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-009-9294-4