Abstract
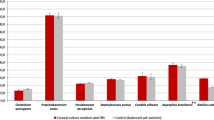
Living tissue engineering for regenerative therapy cannot withstand the usual pharmacopoeia methods of purification and terminal sterilization. Consequently, these products must be manufactured under aseptic conditions at microbiologically controlled environment facilities. This study was proposed to validate BacT/ALERT®3D automated culture system for microbiological control of epithelial cell culture medium (ECCM). Suspensions of the nine microorganisms recommended by the European Pharmacopoeia (Chap. 2.6.27: “Microbiological control of cellular products”), plus one species from oral mucosa and two negative controls with no microorganisms were prepared in ECCM. They were inoculated in FA (anaerobic) and SN (aerobic) culture bottles (Biomérieux, Lyon, France) and incubated in a BacT/ALERT®3D automated culture system. For each species, five sets of bottles were inoculated for reproducibility testing: one sample was incubated at the French Health Products Agency laboratory (reference) and the four others at Cell and Tissue Bank of Lyon, France. The specificity of the positive culture bottles was verified by Gram staining and then subcultured to identify the microorganism grown. The BacT/ALERT®3D system detected all the inoculated microorganisms in less than 2 days except Propionibacterium acnes which was detected in 3 days. In conclusion, this study demonstrates that the BacT/ALERT®3D system can detect both aerobic and anaerobic bacterial and fungal contamination of an epithelial cell culture medium consistent with the European Pharmacopoeia chapter 2.6.27 recommendations. It showed the specificity, sensitivity, and precision of the BacT/ALERT®3D method, since all the microorganisms seeded were detected in both sites and the uncontaminated medium ECCM remained negative at 7 days.

Similar content being viewed by others
References
Braye F, Oddou L, Bertin-Maghit M, Belgacem S, Damour O, Spitalier P, Guillot M, Bouchard C, Gueugniaud PY, Goudeau M, Petit P, Tissot E (2000a) Widely meshed autograft associated with cultured autologous epithelium for the treatment of major burns in children: report of 12 cases. Eur J Pediatr Surg 10:35–40
Braye F, Pascal P, Bertin-Maghit M, Colpart JJ, Tissot E, Damour O (2000b) Advantages of using a bank of allogenic keratinocytes for the rapid coverage of extensive and deep second-degree burns. Med Biol Eng Comput 38:248–252
Braye F, Dumortier R, Bertin-Maghit M, Girbon JP, Tissot E, Damour O (2001) Cultured epidermis for the treatment of severe burns. A 2-year study (18 patients). Ann Chir Plast Esthet 46:599–606
Brecher ME, Means N, Jere CS, Heath D, Rothenberg S, Stutzman LC (2001) Evaluation of an automated culture system for detecting bacterial contamination of platelets: an analysis with 15 contaminating organisms. Transfusion 41:477–482
Dumont LJ, Hay SN, Herschel L, Brantigan B, Houghton J, Elfath MD, Brecher ME (2011) Validation of a microbial detection system for use with ACD-A platelets with PAS III platelet additive solution. Transfusion 51:2219–2227
Dunne WJ, Case L, Isgriggs L, Lublin D (2005) In-house validation of the BACTEC 9240 blood culture system for detection of bacterial contamination in platelet concentrates. Transfusion 45:1138–1142
European Pharmacopeia 7.0 (2010) Section 2.6.27 “Microbiological control of cellular products”, p 190
Gallico GG 3rd, O’Connor NE, Compton CC, Kehinde O, Green H (1984) Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med 311:448–451
Khuu HM, Stock F, McGann M, Carter CS, Atkins JW, Murray PR, Read EJ (2004) Comparison of automated culture systems with a CFR/USP-compliant method for sterility testing of cell-therapy products. Cytotherapy 6:183–195
Khuu HM, Patel N, Carter CS, Murray PR, Read EJ (2006) Sterility testing of cell therapy products: parallel comparison of automated methods with a CFR-compliant method. Transfusion 46:2071–2082
Kielpinski G, Prinzi S, Duguid J, du Moulin G (2005) Roadmap to approval: use of an automated sterility test method as a lot release test for carticel, autologous cultured chondrocytes. Cytotherapy 7:531–541
Kopp J, Wang GY, Kulmburg P, Schultze-Mosgau S, Huan JN, Ying K, Seyhan H, Jeschke MD, Kneser U, Bach AD, Ge SD, Dooley S, Horch RE (2004) Accelerated wound healing by in vivo application of keratinocytes overexpressing KGF. Mol Ther 10:86–96
Mastronardi C, Perkins H, Derksen P, Den Admirant M, Ramirez-Arcos S (2010) Evaluation of the BacT/ALERT 3D system for the implementation of in-house quality control sterility testing at Canadian blood services. Clin Chem Lab Med 48:1179–1187
McDonald CP, Roy A, Lowe P, Robbins S, Hartley S, Barbara JA (2001) Evaluation of the BacT/Alert automated blood culture system for detecting bacteria and measuring their growth kinetics in leucodepleted and non-leucodepleted platelet concentrates. Vox Sang 81:154–160
Nakamura T, Endo K, Cooper LJ, Fullwood NJ, Tanifuji N, Tsuzuki M, Koizumi N, Inatomi T, Sano Y, Kinoshita S (2003) The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci 44:106–116
Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, Nagai S, Kikuchi A, Maeda N, Watanabe H, Okano T, Tano Y (2004) Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med 351:1187–1196
Rama P, Bonini S, Lambiase A, Golisano O, Paterna P, De Luca M, Pellegrini G (2001) Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation 72:1478–1485
Schwella N, Rick O, Heuft HG, Miksits K, Zimmermann R, Zingsem J, Eckstein R, Huhn D (1998) Bacterial contamination of autologous bone marrow: reinfusion of culture-positive grafts does not result in clinical sequelae during the post-transplantation course. Vox Sang 74:88–94
Thepot A, Morel AP, Justin V, Desanlis A, Thivillier L, Hoffman E, Till M, Accardi R, Tommasino M, Breton P, Hainaut P, Damour O (2010) Evaluation of tumorigenic risk of tissue-engineered oral mucosal epithelial cells by using combinational examinations. Cell Transplant 19:999–1006
Acknowledgments
We thank Biomérieux for lending the BacT/ALERT®3D system for the duration of the study and Vincent Langeron for his assistance in writing the report.
Conflict of interest
The material presented in this article is original research which has not been previously published and has not been submitted for publication elsewhere while under consideration. We certify that we have sufficiently participated in the work to take public responsibility for the appropriateness of the experimental design and method, and the collection, analysis, and interpretation of the data. All authors have reviewed the final version of the manuscript and approve it for publication. All the authors declare no personal or financial conflict of interest and have not entered into any agreement that could interfere with our access to the data on the research, or upon our ability to analyze the data independently, to prepare manuscripts, and to publish them.
Author information
Authors and Affiliations
Corresponding author
Additional information
O. Damour and Céline Auxenfans: co-last authors.
Rights and permissions
About this article
Cite this article
Plantamura, E., Huyghe, G., Panterne, B. et al. Validation of the BacT/ALERT®3D automated culture system for the detection of microbial contamination of epithelial cell culture medium. Cell Tissue Bank 13, 453–459 (2012). https://doi.org/10.1007/s10561-011-9281-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-011-9281-1