Abstract
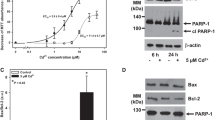
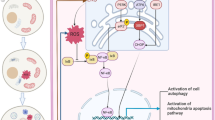
Progressive chronic kidney disease (CKD) is common in lysinuric protein intolerance (LPI), a primary inherited aminoaciduria characterized by massive Lysine excretion in urine. However, by which mechanisms Lysine may cause kidney damage to tubule cells is still not understood. This study determined whether Lysine overloading of human proximal tubular cells (HK-2) in culture enhances apoptotic cell loss and its associated mechanisms. Overloading HK-2 with Lysine levels reproducing those observed in urine of patients affected by LPI (10 mM) increased apoptosis (+30%; p < 0.01 vs.C), as well as Bax and Apaf-1 expressions (+30-50% p < 0.05), while downregulated Bcl-2 (-40% p < 0.05). Apoptosis induced by high Lysine was no longer observed after addition of caspase-9 and caspase-3 inhibitors while caspase-8 inhibitor had no protective effect. High Lysine induced elevations in ROS generation and NADPH oxidase subunits mRNAs (p22 phox +106±23%, p67 phox +108±22% and gp91 phox +75±4% p < 0.05-0.01). In addition, the NADPH oxidase inhibitor DPI prevented both ROS production and apoptosis. Treating HK-2 with antioxidants, such as Cysteine and its analog, N-acetyl-l-cysteine (NAC), rescued the HK-2 from apoptosis induced by Lysine. In summary, our data show that high Lysine in vitro increases the permissiveness of proximal tubule kidney cells to apoptosis by triggering a pathway involving NADPH oxidase signaling. This event may represent a key cellular effect in the increasing the susceptibility of human tubular cells to apoptosis when the tubules cope with a high Lysine load. This effect is instrumental to renal damage and disease progression in patients with LPI.








Similar content being viewed by others
References
Asanuma K, Adachi K, Sugimoto T et al (2006) Effects of lysine-induced acute renal failure in dogs. J Toxicol Sci 31:87–98
Barnes JL, Gorin Y (2011) Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int 79:944–956
Benninga MA, Lilien M, de Koning TJ et al (2007) Renal Fanconi syndrome with ultrastructural defects in lysinuric protein intolerance. J Inherit Metab Dis 30:402–403
Bröer S (2007) Lysinuric protein intolerance: one gene, many problems. Am J Physiol Cell Physiol 293(2):C540–541
Brown DI, Griendling KK (2009) Nox proteins in signal transduction. Free Radic Biol Med 47:1239–1253
Camargo SMR, Bockenhauer D, Kleta R (2008) Aminoacidurias: clinical and molecular aspects. Kidney Int 73:918–925
Capellino S, Montagna P, Villaggio B et al (2008) Hydroxylated estrogen metabolites influences the proliferation of cultured human monocytes: possibile role in synovial tissue hyperplasia. Clin Exp Rheumatol 26:903–909
Carden DL, Granger N (2000) Pathophysiology of ischemia-reperfusion injury. J Pathol 190:255–266
Chillarón J, Font-Llitjós M, Fort I et al (2010) Pathophysiology and treatment of cystinuria. Nat Rev Nephrol 6:424–434
Di Rocco M, Garibotto G, Rossi GA et al (1993) Role of hematological, pulmonary and renal complications in the long-term prognosis of patients with Lysinuric protein intolerance. Eur J Pediatrics 152:437–442
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107:1058–1070
Gill PS, Wilcox CS (2006) NADPH oxidases in the kidney. Antioxid Redox Signal 8:1597–1607
Gorin Y, Block K, Hernandez J et al (2005) Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280:39616–39626
Janicke RU, Sprengart ML, Wati MR et al (1998) Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem 273:9357–9360
Liu F, Wei CC, Wu SJ et al (2009) Apocyanin attenuates tubular apoptosis and tubulointerstitial fibrosis in transgenic mice independent of hypertension. Kidney Int 75:156–166
Lodha S, Dani D, Mehta R et al (2002) Angiotensin II-induced mesangial cell apoptosis: role of oxidative stress. Mol Med 8:830–840
Morais C, Westhuyzen J, Metharom P et al (2005) High molecular weight plasma proteins induce apoptosis and Fas/FasL expression in human proximal tubular cells. Nephrol Dial Transplant 20:50–55
New DD, Block K, Bhandari B et al (2011) IGF-1 increases the expression of fibronectin by Nox-4 dependent AKT phosphorylation in renal tubular epithelial cells. Am J Physiol Cell Physiol in press
Palacin M, Bertran J, Chillaròn J et al (2004) Lysinuric protein intolerance: mechanisms of pathophysiology. Mol Genet Metab 81:S27–37
Palm F, Nangaku M, Fasching A et al (2010) Uremia induces abnormal oxygen consumption in tubules and aggravates chronic hypoxia of the kidney via oxidative stress. Am J Physiol Renal Physiol 299:F380–386
Parenti G, Sebastio G, Strisciuglio P et al (1995) Lysinuric protein intolerance characterized by bone marrow abnormalities and severe clinical course. J Pediatr 126:246–251
Parto K, Kallajoki M, Aho H et al (1994) Pulmonary alveolar proteinosis and glomerulonephritis in lysinuric protein intolerance: case reports and autopsy findings of four pediatric patients. Hum Pathol 25(4):400–407
Racusen LC, Whelton A, Solez K (1985) Effects of lysine and other amino acids on kidney structure and function in the rat. Am J Pathol 120:436–442
Ricci JE, Gottlieb RA, Green DR (2003) Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J Cell Biol 160:65–75
Schreck C, O'Connor PM (2011) NAD(P)H oxidase and renal epithelial ion transport. Am J Physiol Regul Integr Comp Physiol 300:R1023–R1029
Sebastio G, Sperandeo MP, Andria G (2011) Lysinuric protein intolerance: reviewing concepts on a multisystem disease. Am J Med Genet C Semin Med Genet 157:54–62
Sedeek M, Callera G, Montezano A et al (2010) Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299:F1348–1358
Susztak K, Raff AC, Schiffer M et al (2006) Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55:225–233
Tanner LM, Näntö-Salonen K, Niinikoski H et al (2007) Nephropathy advancing to end-stage renal disease: a novel complication of lysinuric protein intolerance. J Pediatr 150:631–634
Thelle K, Christensen EI, Vorum H et al (2006) Characterization of proteinuria and tubular protein uptake in a new model of oral l-lysine administration in rats. Kidney Int 69:1333–1340
Verzola D, Bertolotto MB, Villaggio B et al (2002) Taurine prevents apoptosis induced by high glucose in human tubule renal cells. J Invest Med 50:443–451
Acknowledgements
This study was supported by a Grants from the Ministero dell’Università e della Ricerca Scientifica e Tecnologica and from the Genoa University, Italy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Verena Peters
Competing interest: None declared.
Rights and permissions
About this article
Cite this article
Verzola, D., Famà, A., Villaggio, B. et al. Lysine triggers apoptosis through a NADPH oxidase-dependent mechanism in human renal tubular cells. J Inherit Metab Dis 35, 1011–1019 (2012). https://doi.org/10.1007/s10545-012-9468-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-012-9468-z