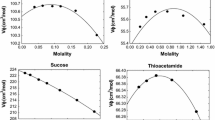
Abstract
The density and compressibility of seawater solutions from 0 to 95 °C have been examined using the Pitzer equations. The apparent molal volumes (X = V) and compressibilities (X = κ) are in the form
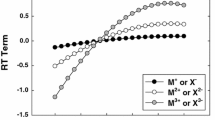
where \( \bar{X}^{0} \) is the partial molal volume or compressibility, I is the ionic strength, m is the molality of sea salt, AX is the Debye–Hückel slope for volume (X = V) or adiabatic compressibility (X = κ s), and g(y) = (2/y 2)[1 − (1 + y) exp(−y)] where y = 2I 0.5. The values of the partial molal volume and compressibility (\( \bar{X}^{0} \)) and Pitzer parameters (β (0)X, β (1)X and C X) are functions of temperature in the form
where a i are adjustable parameters, T is the absolute temperature in Kelvin, and T R = 298.15 K is the reference temperature. The standard errors of the seawater fits for the specific volumes and adiabatic compressibilities are 5.35E−06 cm3 g−1 and 1.0E−09 bar−1, respectively. These equations can be combined with similar equations for the osmotic coefficient, enthalpy and heat capacity to define the thermodynamic properties of sea salt to high temperatures at one atm. The Pitzer equations for the major components of seawater have been used to estimate the density and compressibility of seawater to 95 °C. The results are in reasonable agreement with the measured values (0.010E−03 g cm−3 for density and 0.050E−06 bar−1 for compressibility) from 0 to 80 °C and salinities from 0 to 45 g kg−1. The results make it possible to estimate the density and compressibility of all natural waters of known composition over a wide range of temperature and salinity.






Similar content being viewed by others
References
Ananthaswamy J, Atkinson G (1984) Thermodynamics of concentrated electrolyte mixtures. 4. Pitzer-Debye-Hückel Limiting slopes for water from 0 to 100°C and from 1 atm to 1 kbar. J Chem Eng Data 29:81–87
Connaughton LM, Hershey JP, Millero FJ (1986) The PVT properties of concentrated aqueous electrolytes: V. Densities and apparent molal volumes of the four major sea salts from dilute solution to saturation and from 0 to 100°C. J Solution Chem 15:989–1002
Connaughton LM, Millero FJ, Pitzer KS (1989) Volume changes for mixing the major sea salts: equations valid to ionic strength 3.0 and temperature 95°C. J Solution Chem 18:1007–1017
Dedick E, Stade D, Sotolongo S, Hershey JP, Millero FJ (1990) The PVT properties of concentrated aqueous electrolytes. IX. The volume properties of KCl and K2SO4 and their mixtures with NaCl and Na2SO4 as a function of temperature. J Solution Chem 19:353–374
Hershey JP, Sotolongo S, Millero FJ (1983) Densities and compressibilities of aqueous sodium carbonate and bicarbonate from 0 to 45°C. J Solution Chem 12:233–254
Hershey JP, Damesceno R, Millero FJ (1984) Densities and compressibilities of aqueous HCl and NaOH from 0 to 45°C. The effect of pressure on the ionization of water. J Solution Chem 13:825–848
Krumgalz BS, Pogorelsky R, Iosilevskii YaA, Weiser A, Pitzer KS (1994) Ion interaction approach for volumetric calculations for solutions of single electrolytes at 25°C. J Solution Chem 23:849–875
Krumgalz BS, Pogorelsky R, Pitzer KS (1995) Ion interaction approach to calculations of volumetric properties of aqueous multiple-solute electrolyte solutions. J Solution Chem 24:1025–1038
Krumgalz BS, Pogorelsky R, Pitzer KS (1996) Volumetric properties of single aqueous electrolytes from zero to saturation concentration 298.15°K represented by Pitzer’s ion interaction equations. J Phys Chem Ref Data 25:663–689
Krumgalz BS, Pogorelsky R, Sokolov A, Pitzer KS (2000) Volumetric ion interaction parameters for single-solute aqueous electrolyte solutions at various temperatures. J Phys Chem Ref Data 29:1123–1140
Lee K, Kim TW, Byrne R, Millero FJ, Feely RA, Liu YM (2010) The universal ratio of boron to chlorinity for the North Pacific and North Atlantic oceans. Geochim Cosmochim Ac 74:1801–1811
Millero FJ (1983) Influence of pressure on chemical processes in the sea. In: Riley JP, Chester R (eds) Chemical oceanography, vol 8. Academic Press, London, pp 1–88
Millero FJ (2006) Chemical oceanography, 3rd edn. CRC Press, Boca Raton
Millero FJ, Huang F (2009) The density of seawater as a function of salinity (5 to 70 g kg−1) and temperature (273.15 to 363.15 K). Ocean Sci 5:91–100
Millero FJ, Huang F (2011) The compressibility of seawater from 0 to 95°C at 1 atm. Marine Chem 126:149–154
Millero FJ, Lampreia MI (1985) The PVT properties of concentrated aqueous electrolytes. IV. Changes in the compressibilities of mixing the major sea salts at 25 °C. J Solution Chem 14:853–864
Millero FJ, Pierrot D (2005) The thermochemical properties of seawater fit to the Pitzer equations. Marine Chem 94:81–99
Millero FJ, Ward GK, Chetirkin PV (1977) Relative sound velocities of sea salts at 25 °C. J Acoust Soc Am 61:1492–1498
Millero FJ, Vinokurova F, Fernandez M, Hershey JP (1987) PVT properties of concentrated electrolytes. VI. The speed of sound and apparent molal compressibilities of NaCl, Na2SO4, MgCl2 and MgSO4 solutions from 0 to 100 °C. J Solution Chem 16:269–284
Millero FJ, Feistel R, Wright DG, McDougall TJ (2008) The composition of standard seawater and the definition of the reference-composition salinity scale. Deep Sea Res Part I 55:50–72
Monnin C (1987) Densities and apparent molal volumes of aqueous CaCl2 and MgCl2 solutions. J Solution Chem 16:1035–1048
Monnin C (1989) An ion interaction model for the volumetric properties of natural waters: density of the solution and partial molal volumes of electrolytes to high concentration at 25 °C. Geochim Cosmochim Acta 53:1177–1188
Monnin C (1990) The influence of pressure on the activity coefficients of the solutes and on the solubility of minerals in the system Na–Ca–Cl–SO4–H2O to 2008C and 1 kbar, and to high NaCl concentration. Geochim Cosmochim Acta 54:3265–3282
Pierrot D, Millero FJ (2000) The apparent molal volume and compressibility of seawater fit to the Pitzer equations. J Solution Chem 29:719–742
Pitzer KS (1991) Ion interaction approach: theory and data correlation. In: Pitzer KS (ed) Activity coefficients in electrolyte solutions, 2nd edn. CRC Press, Boca Raton, pp 75–153
Acknowledgments
We wish to acknowledge the support of the oceanographic section of the National Science Foundation, for our Marine Physical Chemical Studies over the years. We also want to acknowledge the work of my dear friend Boris Krumgalz who summarized the Pitzer volume parameters for the major salts in seawater in his 2000 paper.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
See Table 6.
Appendix 2
See Table 7.
Appendix 3
See Table 8.
Appendix 4
See Table 9.
Rights and permissions
About this article
Cite this article
Rodriguez, C., Millero, F.J. Estimating the Density and Compressibility of Seawater to High Temperatures Using the Pitzer Equations. Aquat Geochem 19, 115–133 (2013). https://doi.org/10.1007/s10498-012-9183-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-012-9183-2