Abstract
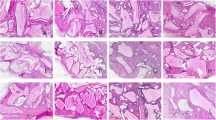
The purpose of this study was to analyze histologically the effect of low-level laser therapy (LLLT) in combination with bisphosphonate on bone healing in surgically created critical size defects (CSD) in rat calvaria. One hundred Wistar female rats sham operated (sham) and ovariectomized (Ovx) were maintained untreated for 1 month to allow for the development of osteopenia in the Ovx animals. A CSD was made in the calvarium of each rat, and the animals were divided into five groups according to following treatments: (1) sham rats (control), (2) Ovx rats, (3) Ovx rats treated with LLLT, (4) Ovx rats treated with bisphosphonate, and (5) Ovx rats treated with bisphosphonate and LLLT. Groups 4 and 5 were irrigated with 1 ml of bisphosphonate, and groups 3 and 5 were submitted to LLLT (GaAlAs), 660 nm, 24 J, and 0.4285 W/cm2 on the CSD. Ten animals of each treatment were killed at 30 and 60 days. Histomorphometric assessments, using image analysis software, and histological analyses were performed. No defect was completely regenerated with the bone. Histometrically, it can be observed that groups 3 (37.49 ± 1.94%, 43.11 ± 2.39%) and 5 (35.05 ± 1.57%, 41.07 ± 1.89%) showed a significant bone neoformation when compared to groups 1 (16.81 ± 1.57%, 27.54 ± 1.49%), 2 (11.68 ± 0.98%, 22.51 ± 1.05%), and 4 (14.62 ± 1.70%, 25.67 ± 1.41%) in all experimental periods (P < 0.05). It was possible to conclude that the LLLT associated or not with bisphosphonate treatment was effective for stimulating bone formation in CSD in the calvaria of rats submitted to ovariectomy.




Similar content being viewed by others
References
Mangolas SC (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137
Gali JC (2001) Osteoporose. Acta Ortop Bras 9:53–62
Consensus Development Conference on Osteoporosis (1993) Am J Med 95A:5S–16S
Friedlander AH (2002) The physiology, medical management and oral implications of menopause. J Am Dent Assoc 133:73–81
Botell M (2001) Osteoporosis en la menopausia, prevención y estratégias terapêuticas atuales. Rev Cub Obst Ginecol 27:199–204
Park-Wyllie LY, Mamdani MM, Juurlink DN, Hawker GA, Gunraj N, Austin PC, Whelan DB, Weiler PJ, Laupacis A (2011) Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 305:783–789
Manolagas SC (2010) From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev 31:266–300
Menezes AM, Rocha FA, Chaves HV, Carvalho CB, Ribeiro RA, Brito GAJ (2005) Effect of sodium alendronate on alveolar bone resorption in experimental periodontitis in rats. Periodontol 2000 76:1901–1909
Thomopoulos S, Matsuzaki H, Zaegel M, Gelberman RH, Silva MJ (2007) Alendronate prevents bone loss and improves tendon-to-bone repair strength in a canine model. J Orthop Res 25:473–479
Grey A, Bolland MJ, Wattie D, Horne A, Gamble G, Reid IR (2009) The antiresorptive effects of a single dose of zoledronate persist for two years: a randomized, placebo-controlled trial in osteopenic postmenopausal women. J Clin Endocrinol Metab 94:538–544
Luger EJ, Rochkind S, Wollman Y, Kogan G, Dekel S (1998) Effect of low power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers Surg Med 22:97–102
Guzzardella GA, Fini M, Torricelli P, Giavaresi G, Giardino R (2002) Laser stimulation on bone defect healing: an in vitro study. Lasers Med Sci 17:216–220
Freitas IGF, Baranauskas V, Cruz-Höfling MA (2000) Laser effects on osteogenesis. Appl Surf Sci 154–155:548–554
Trelles MA, Mayayo E (1987) Bone fracture consolidates faster with low-power laser. Lasers Surg Med 7:36–45
Garavello-Freitas I, Baranauskas V, Joazeiro PP, Padovani CR, Dal Pai-Silva M, Da Cruz-Höfling MA (2003) Low-power laser irradiation improves histomorphometrical parameters and bone matrix organization during tibia wound healing in rats. J Photochem Photobiol 70:81–89
Karu T, Pyatibrat L, Kalendo G (1995) Irradiation with He–Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol 27:219–223
Alghamdi KM, Kumar A, Moussa NA (2011) Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci 27(1):237–249
Dortbudak O, Haas R, Mailath-Pokorny G (2002) Effect of low power laser irradiation on bony implant sites. Clin Oral Implants Res 13:288–292
Nissan J, Assif D, Gross MD, Yaffe A, Binderman I (2006) Effect of low intensity laser irradiation on surgically created bony defects in rats. J Oral Rehabil 33:619–924
Lunger EJ, Rochkind S, Wollman Y, Kogan G, Dekel S (1998) Effect of low power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers Surg Med 22:97–102
Barushka O, Yaakobi T, Oron U (1995) Effect of low-energy laser (He–Ne) irradiation. Bone 16:47–55
Bosch C, Melsen B, Vargervik K (1998) Importance of the critical-size bone defect in testing bone-regenerating materials. J Craniofac Surg 9:310–316
Furlaneto FA, Nagata MJ, Fucini SE, Deliberador TM, Okamoto T, Messora MR (2007) Bone healing in critical-size defects treated with bioactive glass/calcium sulfate: a histologic and histometric study in rat calvaria. Clin Oral Implants Res 18:311–318
Melo LG, Nagata MJ, Bosco AF, Ribeiro LL, Leite CM (2005) Bone healing in surgically created defects treated with either bioactive glass particles, a calcium sulfate barrier, or a combination of both materials. A histological and histometric study in rat tibias. Clin Oral Implants Res 16:683–691
Frost HM, Jee WSS (1992) On the rat model of human osteopenias and osteoporosis. Bone Miner 18:227–236
Pytlik M, Janiec W, Misiarz-Myrta M, Gubata I (2003) Effects of simvastatin on the development of osteopenia caused by ovariectomy in rats. Pol J Pharmacol 55:63–71
Pytlik M, Kaczmarczyk-Sedlak I, Sliwiński L, Janiec W, Rymkiewicz I (2004) Effect of concurrent administration of alendronate sodium and retinol on development of changes in histomorphometric parameters of bones induced by ovariectomy in rats. Pol J Pharmacol 56:571–579
Houde N, Chamoux E, Bisson M, Roux S (2009) Transforming growth factor-beta1 (TGF-beta1) induces human osteoclast apoptosis by up-regulating Bim. J Biol Chem 284:23397–23404
Gutteridge DH, Retallack RW, Ward LC, Price RI, Stewart GO, Stuckey BG, Prince RL, Kent GN, Bhagat CI, Thompson RI, Nicholson GC (2003) Bone density changes in Paget’s disease 2 years after iv pamidronate: profound, sustained increases in pagetic bone with severity-related loss in forearm nonpagetic cortical bone. Bone 32:56–61
Igarashi K, Adachi H, Mitani H, Shinoda H (1996) Inhibitory effect of the topical administration of a bisphosphonate (risendronate) on root resorption incident to orthodontic tooth movements in rats. J Dent Res 75:1644–1649
Levin L, Bryson EC, Caplan D, Trope M (2001) Effect of topical alendronate on root resorption of dried replanted dog teeth. Dent Traumatol 17:120–126
Berggreen E, Sae-Lim V, Bletsa A, Heyeraas KJ (2001) Effect of denervation on healing after tooth replantation in the ferret. Acta Odontol Scand 59:379–385
Bohic S, Rey C, Legrand A, Sfihi H, Rohanizadeh R, Martel C, Barbier A, Daculsi G (2000) Characterization of the trabecular rat bone mineral: effect of ovariectomy and bisphosphonate treatment. Bone 26:341–348
Sliwiński L, Janiec W, Pytlik M, Folwarczna J, Kaczmarczyk-Sedlak I, Pytlik W, Cegieła U, Nowińska B (2004) Effect of administration of alendronate sodium and retinol on the mechanical properties of the femur in ovariectomized rats. Pol J Pharmacol 56:817–824
Kana JS, Hutschenreiter G, Haina D, Waidelich W (1981) Effect of low-power density laser radiation on healing of open skin wound in rats. Arch Surg 116:293–296
Boulton M, Marshall J (1986) He–Ne laser stimulation of human fibroblast proliferation and attachment in vitro. Lasers Life Sci 1:123–134
Schultz RJ, Krishnamurthy S, Thelmo W, Rodriguez JE, Harvey G (1985) Effects of varying intensities of laser energy on articular cartilage. Lasers Surg Med 5:557–588
Honmura A, Yanase M, Obata J, Haruki E (1992) Therapeutic effect of Ga-Al-As diode laser irradiation on experimentally induced inflammation in rats. Lasers Surg Med 12:441–449
Anders JJ, Borke RC, Woolery SK, Van de Merwe WP (1993) Low power laser irradiation alters the rate of regeneration of the rat facial nerve. Lasers Surg Med 13:72–82
Dortbudak O, Hass R, Pokorny G (2000) Biostimulation of bone marrow cells with a diode soft laser. Clin Oral Implants Res 11:540–545
Ueda Y, Shimizu N (2003) Effects of pulse frequency of low-level laser therapy (LLLT) on bone nodule formation in rat calvarial cells. J Clin Laser Med Surg 21:271–277
Ozawa Y, Shimizu N, Kariya G, Abiko Y (1998) Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone 22:347–354
Coombe AR, Ho CTG, Darendeliler MA, Hunter N, Philips JR, Chapple CC, Yum LW (2001) The effects of low-level laser irradiation on osteoblastic cells. Clin Orthop Res 4:3–14
Silva Júnior AN, Pinheiro AL, Oliveira MG, Weismann R, Ramalho LM, Nicolau RA (2002) Computerized morphometric assessment of the effect of low-level laser therapy on bone repair: an experimental animal study. J Clin Laser Med Surg 20:83–87
Kipshidze N, Nikolaychic V, Keelan MH, Shankar LR, Khanna A, Kornowski R, Leon M, Moses J (2001) Low power helium: neon laser irradiation enhances production of vascular endothelial growth factor and promotes growth of endothelial cells in vitro. Lasers Surg Med 28:355–364
Diniz JS, Nicolau RA, de Melo ON, do Carmo Magalhães F, de Oliveira Pereira RD, Serakides R (2009) Effect of low-power gallium-aluminum-arsenium laser therapy (830 nm) in combination with bisphosphonate treatment on osteopenic bone structure: an experimental animal study. Lasers Med Sci 24:347–352
Pyczek M, Sopala M, Dabrowski Z (1994) Effect of low energy laser power on the bone marrow of the rat. Folia Biol 42:151–156
Takagi K, Urist MR (1982) The reaction of the dura to bone morphogenetic protein (BMP) in repair of skull defects. Ann Surg 196:100–109
Acknowledgments
Juliana Mendonça da Conceição received a scholarship from the São Paulo State Foundation for Research (FAPESP: 2007/55072-5). This paper is attributed to the Department of Periodontology, Araçatuba Dental School, São Paulo State University (UNESP) Araçatuba, São Paulo, Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garcia, V.G., da Conceição, J.M., Fernandes, L.A. et al. Effects of LLLT in combination with bisphosphonate on bone healing in critical size defects: a histological and histometric study in rat calvaria. Lasers Med Sci 28, 407–414 (2013). https://doi.org/10.1007/s10103-012-1068-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1068-5