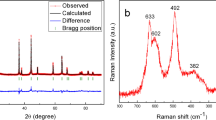
Abstract
The effect of different membranes and aluminum current collectors on the initial coulombic efficiency of LiNi0.5Mn1.5O4/Li was investigated, and the cycling performance at different rates and temperatures and the storage performance at 60 °C for a week are discussed for LiNi0.5Mn1.5O4/Li. The results show that the lower initial coulombic efficiency is associated with the lower decomposition voltage of the commercial membrane and electrolyte, and the instability of aluminum current collector under the higher voltage. In addition, both versions of LiNi0.5Mn1.5O4 can deliver about 115 mA h g−1 of initial discharge capacity at 1 C at 25 °C and 60 °C; however, it retains only 61.57 % of its initial capacity after the 130th cycles at 60 °C, which is much lower than the 94.46 % rate observed for LiNi0.5Mn1.5O4 at 25 °C, and the cycling performance of the material at 1 C is better than that at 0.5 C. Meanwhile, the initial discharge capacity at 0.1 C after storing at 60 °C is 119.3 mA h g−1, which is only a little lower than 121.5 mA h g−1 recorded before storing; moreover, the spinel structure and surface state of LiNi0.5Mn1.5O4 after storing at 60 °C has not been changed basically. These results indicate that the electrochemical stability of electrolyte is also related to the temperature. The serious capacity fading of LiNi0.5Mn1.5O4 at 60 °C is attributed to the severe oxidation decomposition and the thermal decomposition in the range of cut-off voltage of the materials, and then the decomposition products interact with active materials to form a solid interface phase, leading to the larger electrode polarization and irreversible capacity loss. Meanwhile, the worse cycling performance at 0.5 C than that at 1 C is attributed to the longer interaction time between the electrolyte and the active materials. However, the storage performance of LiNi0.5Mn1.5O4 corresponds to the thermal stability of electrolyte to a certain extent.











Similar content being viewed by others
References
Sun YK, Hong KJ, Prakash J, Amine K (2002) Eletrochem Commun 4:344–348
Santhanam R, Rambabu B (2010) J Power Sources 195:5442–5451
Shin DW, Manthiram A (2011) Eletrochem Commun 13:1213–1216
Liu J, Manthiram A (2009) J Phys Chem C 113:15073–15079
Zhong GB, Wang YY, Zhang ZC, Chen CH (2011) Electrochim Acta 56:6554–6561
Shi JY, Yi CW, Kim K (2010) J Power Sources 195:6860–6866
Arrebola JC, Caballero A, Hernán L, Morales J (2008) J Power Sources 180:852–858
Liu DL, Bai Y, Zhao S, Zhang WF (2012) J Power Sources 219:333–338
Zhao GY, Lin YB, Zhou T, Lin Y, Huang YD, Huang ZG (2012) J Power Sources 215:63–68
Liu GQ, Wen L, Liu YM (2010) J Solid State Electrochem 14:2191–2202
Liu GQ, Yuan WS, Liu GY, Tian YW (2009) J Alloy Compd 484:567–569
Wu HM, Tu JP, Chen XT, Shi DQ, Zhao XB, Cao GS (2006) Electrochim Acta 51:4148–4152
Zhang X, Zheng H, Battaglia V, Axelbaum RL (2011) Proc Combust Inst 33:1867–1874
Chen XL, Xu W, Xiao J, Engelhard MH, Ding F, Mei DH, Hu DH, Zhang J, Zhang JG (2012) J Power Sources 213:160–168
Markovsky B, Talyossef Y, Salitra G, Aurbach D, Kim HJ, Choi S (2004) Electrochem Commun 6:821–826
Aurbach D, Markovsky B, Talyossef Y, Salitra G, Kim HJ, Choi S (2006) J Power Sources 162:780–789
Hugues D, Dominique D, Yaser AL, Isobel JD (2011) J Electrochem Soc 158:A537–A545
Kostecki R, McLarnon F (2004) Electrochem Solid State Lett 7:A380–A383
Talyosef Y, Markovsky B, Salitra G, Aurbach D, Kim HJ, Choi S (2005) J Power Sources 146:664–669
Myung ST, Hitoshi Y, Sun YK (2011) J Mater Chem 21:9891–9911
Yoon TH, Park SJ, Mun JY, Ryu JH, Choi WC, Kang YS, Park JH, Oh SM (2012) J Power Sources 215:312–316
Surendra KM, Nancy JD, James OK, Jagjit N (2012) J Electrochem Soc 159:A1652–A1658
Acknowledgment
This work was financially supported by the Major Special Programs of Science and Technology of Hunan Province (2011FJ1005) and Innovative Project of Graduate in Hunan Province (CX2011B104), which are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, X., Li, X., Wang, Z. et al. Comprehensive reinvestigation on the initial coulombic efficiency and capacity fading mechanism of LiNi0.5Mn1.5O4 at low rate and elevated temperature. J Solid State Electrochem 17, 1029–1038 (2013). https://doi.org/10.1007/s10008-012-1963-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-012-1963-5