Abstract
Solubility equilibria between solid salts, salt hydrates, and water have been analyzed on a thermodynamic basis. Fitting equations with three or four adjustable parameters suffice to describe the temperature dependence of solubility adequately when the latter is expressed in ionic (species) mole fractions. Phase diagrams, temperature versus mole fractions, summarize and deal concisely with equilibrium solubility properties of the systems. Comparison of experimental and estimated ratios of slopes to liquidus curves intersecting at isobaric invariants, such as, for example, eutectic or peritectic points, provides a thermodynamic consistency test.
Graphical Abstract



Similar content being viewed by others
References
Eysseltová J, Timofeevna Orlova V (2010) IUPAC-NIST solubility data series, 89. Alkali metal nitrates, part 1: lithium nitrate. J Phys Chem Ref Data 39:033104
Gamsjäger H, Magalhães MCF, Königsberger E, Sawada K, Churagulov BR, Schmidt P, Zeng D (2011) IUPAC-NIST solubility data series, 92. Metal carbonates, part 1: solubility and related thermodynamic quantities of cadmium(II) carbonate in aqueous systems. J Phys Chem Ref Data 40:043104/1-26/
Mioduski T, Gumiński C, Zeng D (2012) IUPAC-NIST solubility data series, 94. Rare earth metal iodides and bromides in water and aqueous systems, part 1: Iodides. J Phys Chem Ref Data 41:013104
Lorimer JW, Cohen-Adad R (2003) Thermodynamics of Solubility. In: Hefter GT, Tomkins RPT (eds) Experimental Determination of Solubilities. John Wiley & Sons, Chichester, UK; sect 3, p 22 and sect 6, p 53
Gamsjäger H, Lorimer JW, Salomon M, Shaw DG, Tomkins RPT (2010) Pure Appl Chem 82:1137 (Tab. 3, Equation 30)
Gamsjäger H, Lorimer JW, Salomon M, Shaw DG, Tomkins RPT (2010) J Phys Chem Ref Data 39:023101 (Tab. 3, Equation 30)
Clarke ECW, Glew DN (1966) Trans Faraday Soc 62:539
Gumiński C, Voigt H, Zeng D (2011) Monatsh Chem 142:211
Pelton AD (1988) Metal Mater Trans A 19A:819
Gamsjäger H, Lorimer JW, Scharlin P, Shaw DG (2008) Pure Appl Chem 80:233; www.iupac.org/publications/PAC. doi:10.1351/pac200880020233
Wagman DD, Evans WH, Parker VB, Schumm RH, Halow I, Bailey SM, Churney KL, Nuttall RL (1982) The NBS Tables of Chemical Thermodynamic Properties. J Phys Chem Ref Data 11(Suppl):2
Cox JD, Wagman DD, Medvedev VA (1989) CODATA key values for thermodynamics. Hemisphere Publ Corp, New York
Scatchard G, Jones PT, Prentiss SS (1932) J Am Chem Soc 54:290
Washburn EW (ed) (1929) International critical tables of numerical data, physics, chemistry and technology, vol 5. McGraw-Hill, New York p 95–113
Nedeljković L (1968) Z Anorg Allg Chem 357:103
Schürmann E, Nedeljkovic L (1970) Ber Bunsen-Ges Phys Chem 74:462
Guion J, Sauzade JD, Laügt M (1983) Thermochim Acta 67:167
Sinistri C, Franzosini P (1963) Ric Sci Parte 2 Sez A 3:419
Zeng D, Ming J, Voigt W (2008) J Chem Thermodyn 40:232
Campbell AN, Bailey RA (1958) Can J Chem 36:18
Acknowledgments
We are grateful to the Solubility Data group in general and the IUPAC Subcommittee on Solubility and Equilibrium Data in particular for many fruitful discussions before, during, and after the International Symposia on Solubility Phenomena and Related Equilibrium Processes.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
The slope ratio for a peritectic point has been given in Eq. (25). Equations for other cases are given here.
Slope ratio at a eutectic point 1 (freezing curve and solubility curve intersect)
When experimental and theoretical slopes neglecting the f terms differ considerably but their ratios agree, the following relationship must hold:
A quite similar result is obtained when the slope ratio \( \frac{{\sigma_{\text{ice/sln}} }}{{\sigma_{\text{sln/SrW}} }} \) is estimated by Eq. (38), the enthalpy version of Eq. (36). Again agreement between the experimental and theoretical slope ratio is much better than between experimental and theoretical individual slopes.
Slope ratio at a eutectic point 2 (solubility curves of anhydrous solid and salt hydrate intersect)
In this case the binary compound has a stable melting point, and the intersection occurs on the high mole fraction side of the composition of the binary compound.
When experimental and theoretical slopes neglecting the f terms differ considerably but their ratios agree at least approximately, the error is partly compensated by Eqs. (40) and (41), which are analogous to Eqs. (36) and (37):
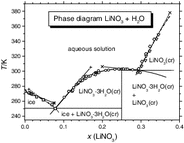
For the system LiNO3 + H2O Eq. (42), the enthalpy version of Eq. (40) is not applicable, because \( \Updelta_{\text{sln}} H_{\text{S}}^{{-}\!\!\!\!\hbox{o}} \) is exothermic (see Table 1), predicting the same sign for \( \sigma_{\text{sln/SrW}} \) and \( \sigma_{\text{sln/S}} \).
Rights and permissions
About this article
Cite this article
Gamsjäger, H., Lorimer, J.W. & Gamsjäger, E. Thermodynamic analysis of solubility data 1: phase diagrams of systems salt hydrate + water. Monatsh Chem 144, 103–112 (2013). https://doi.org/10.1007/s00706-012-0876-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-012-0876-4